[Assoziation zwischen Interleukin-1α und Parodontitis bei Indern: ein narratives Review]
Urvi Vashistha 1Nitik Baisoya 1
Pranav Bansal 1
Pranav Trishal 1
Ruchi Pandey 2
1 Manav Rachna Dental College, School of Dental Sciences, MRIIRS, Faridabad, Haryana, India
2 Department of Periodontology, Manav Rachna Dental College, School of Dental Sciences, MRIIRS, Faridabad, Haryana, India
Zusammenfassung
Hintergrund: Die Ätiologie der Parodontitis ist multifaktoriell und beinhaltet Wechselwirkungen zwischen bakteriellen Erregern, Immunantwort des Wirts und Umweltfaktoren. Unter den Wirtsimmunfaktoren wurde Interleukin-1 alpha (IL-1α) mit der Pathogenese der Parodontitis in Verbindung gebracht. Viele Studien haben versucht, den Zusammenhang zwischen IL-1α und Parodontitis in verschiedenen Bevölkerungsgruppen weltweit zu ermitteln. In der indischen Bevölkerung sind die Erkenntnisse jedoch begrenzt. Daher zielt die Studie darauf ab, Daten über die genetische Assoziation zwischen IL-1α Polymorphismen und Parodontitis bei Indern zu analysieren.
Ziel der Untersuchung war die Analyse von Forschungsdaten über den Zusammenhang zwischen IL-1α Polymorphismen (+4.845 und –889) und verschiedenen Formen der Parodontitis in der indischen Bevölkerung
Methode: Es wurden nur englischsprachige Fall-Kontroll- und Querschnittsstudien aus Indien berücksichtigt, die sich mit dem Zusammenhang zwischen IL-1α und Parodontitis befassen. Für die Suche wurden PubMed, Medline, Web of Science, Cochrane-basierte Reviews, Scopus und Google Scholar verwendet.
Ergebnisse: Die Ergebnisse zeigen ein gemischtes Muster von Assoziationen zwischen diesem Polymorphismus und Parodontitis in verschiedenen Regionen Indiens.
Schlussfolgerung: Der Zusammenhang zwischen Parodontitis und IL-1α-Polymorphismus ist bei Indern nicht belegt.
Schlüsselwörter
Parodontitis, Inder, Interleukin-1 alpha Polymorphismus
Introduction
Periodontitis is governed by the environment, local factors, genetic makeup and phenotype of an individual [1]. The pervasiveness of periodontitis in Indians averages between 63% to 89% across different age groups [2]. Immunity plays a decisive role in maintaining homeostat is during bacterial infection in periodontitis [3]. The predominant cytokines released belong to interleukins, mainly Interleukin-1 alpha (IL-1α) and Interleukin 1β (IL-1β).
The expression of IL-1α is influenced by a gene located on chromosome 2q12-21; its locus is at –889 and +4845 [4]. IL-1α is a cytokine primarily secreted by activated resident gingival cells in response to bacterial challenges [5]. It contributes to the devastation of periodontal tissues by playing a critical part in the initiation and continuation of the inflammatory response. It has been demonstrated that IL-1α increases the expression of other inflammatory mediators, including prostaglandins and chemokines, and stimulates the synthesis of matrix metalloproteinases directly influencing the breakdown of extracellular matrix [6]. Moreover, IL-1α initiates osteoclastic pursuit, thereby increasing bone destruction [7].
Understanding the function of IL-1α in the pathogenesis of periodontitis among the diverse Indian population is critical, since polymorphism can increase the prevalence of disease under disease-promoting environmental conditions, thereby governing the expression of IL1α. However, the research done on the Indian population is limited.
The incidence of periodontitis differs in India throughout socioeconomic categories and geographical areas, indicating the possible impact of both environmental and genetic variables on disease susceptibility [2]. Furthermore, the genetic diversity of the Indian population may influence the production and control of inflammatory mediators such as IL-1α [8].
The aim of the current systematic review was to find the factors associated with IL-1α in causing periodontitis among Indians.
Method
Case control and cross-sectional Indian studies on the association of IL-1α with periodontitis, available as full-length articles in English, were included. Reviews, case reports, and editorials were excluded. An extensive literature search was carried out on electronic databases, i.e., PubMed, Medline, Web of Science, Cochrane based reviews, Scopus, and Google Scholar. Medical Subject Headings (MeSH) terminology and pertinent keywords were combined in the search strategy, included “interleukin-1 alpha”, “IL-1α”, “polymorphism”, “periodontitis”, “chronic periodontitis”, “aggressive periodontitis”, and “India” or “Indian population”. Eligible studies were retrieved and assessed for inclusion by the same two independent reviewers. The review is registered in open science framework and can be cited using osf.iordek/5. To gather pertinent data from the included studies, a standard data extraction form was employed. The following information was extracted:
- Study characteristics (author, study design, year of publication, and Indian area)
- Participant attributes (gender, age, and sample size) (see Table 1 [Tab. 1])
Table 1: Association of IL-1α with demographics, disease cases and single nucleotide polymorphisms (SNPs)
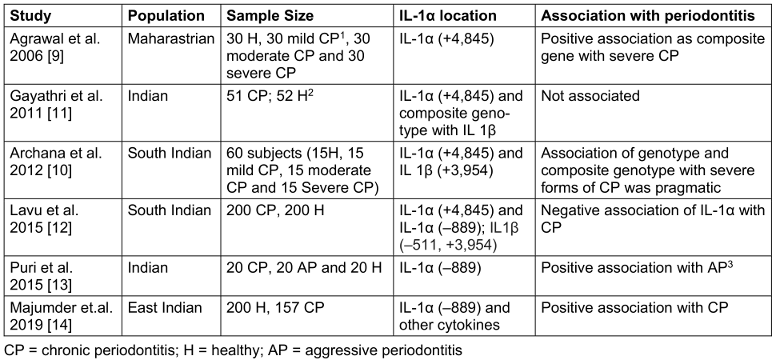
Results
The included studies were conducted across different regions of India. A total of 1,080 subjects were studied, including periodontally affected as well as periodontally healthy individuals. The participants in the included studies were adults of varying age groups, with a mean age of 42 years (Table 1 [Tab. 1]).
Two studies reported a positive association between the IL-1α (+4,845) polymorphism and periodontitis [9], [10], whereas the other studies showed a negative association [11], [12]. Two studies investigated the IL-1α (–889) polymorphism and found a significant correlation [13], [14] (Table 1 [Tab. 1]).
Discussion
The link between IL-1α polymorphisms and periodontitis in the Indian population remains complex and heterogeneous, influenced by factors such as genetic diversity, environmental exposures, and disease subtypes. Several studies highlighted the presence of distinct genetic subgroups within the Indian population, influenced by factors such as geographic location, endogamy, and historical migration patterns [15].
This genetic heterogeneity established through various GWAS studies could potentially influence the distribution and frequencies of IL-1a polymorphisms, as well as their functional implications in the pathogenesis of periodontitis for e.g. in the study by Munz et al. where in a pooled data, a locus at SIGLEC5 (sialic acid binding Ig-like lectin 5) and a chromosomal area downstream of the DEFA1A3 locus (defensin alpha 1–3) were linked to both forms of disease characteristics and were significantly related with periodontitis at the genome-wide level and other similar studies [16], [17], [18], [19], [20], [21], [22].
Environmental factors, including smoking, diet, and oral hygiene practices, are well-established risk factors for periodontitis [23]. These factors may also modulate the expression and activity of IL-1α, thereby influencing its role in the disease process. The included studies did not consistently report on environmental exposures of the study participants, which could contribute to the heterogeneity found. Future studies should consider incorporating environmental factors as potential confounders or affect modifiers in their analyses.
The association between IL-1α polymorphisms and periodontitis subtypes, such as chronic and aggressive forms, was explored in some of the included studies (Table 1 [Tab. 1]). The pathogenesis and immunological mechanisms underlying these subtypes may differ, potentially influencing the role of IL-1α in the disease process influenced by genotype [24]. For instance, aggressive periodontitis is thought to have a stronger genetic component than does chronic periodontitis [25]. Varying associations observed between IL-1α polymorphisms and periodontitis subtypes could reflect these underlying differences in disease mechanisms.
Limitations and future directions
This study had certain limitations, including the potential for publication bias and the moderate to high heterogeneity observed among the included studies. Future investigations should focus on addressing these limitations by conducting more comprehensive, well-designed studies with standardized methodologies, encompassing reporting of participant characteristics, environmental exposures, and genetic data.
Conclusion
The current systematic review failed to find an association of IL-1α polymorphism with periodontitis due to paucity of supporting studies in literature. Nevertheless, despite the heterogeneity of the findings, the identification of specific IL-1α polymorphisms associated with periodontitis in the Indian population could have potential clinical implications. These polymorphisms could serve as potential genetic markers for risk assessment and early disease detection, particularly in high-risk individuals or populations [26]. Furthermore, understanding the involvement of IL-1α in the pathologic process of periodontitis could guide the development of targeted therapeutic interventions, such as anti-inflammatory agents or personalized treatment strategies tailored to the genetic profiles of individuals or specific population groups [27].
Notes
Competing interests
The authors declare that they have no competing interests.
References
[1] Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020 Jun;83(1):26-39. DOI: 10.1111/prd.12297[2] Shaju JP, Zade RM, Das M. Prevalence of periodontitis in the Indian population: A literature review. J Indian Soc Periodontol. 2011 Jan;15(1):29-34. DOI: 10.4103/0972-124X.82261
[3] Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers. 2017 Jun 22;3:17038. DOI: 10.1038/nrdp.2017.38
[4] Modi WS, Masuda A, Yamada M, Oppenheim JJ, Matsushima K, O'Brien SJ. Chromosomal localization of the human interleukin 1 alpha (IL-1 alpha) gene. Genomics. 1988 May;2(4):310-4. DOI: 10.1016/0888-7543(88)90019-5
[5] Pan W, Wang Q, Chen Q. The cytokine network involved in the host immune response to periodontitis. Int J Oral Sci. 2019 Nov 5;11(3):30. DOI: 10.1038/s41368-019-0064-z
[6] Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014 Feb;64(1):57-80. DOI: 10.1111/prd.12002
[7] Lee YM, Fujikado N, Manaka H, Yasuda H, Iwakura Y. IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol. 2010 Oct;22(10):805-16. DOI: 10.1093/intimm/dxq431
[8] Ali M, Liu X, Pillai EN, Chen P, Khor CC, Ong RT, Teo YY. Characterizing the genetic differences between two distinct migrant groups from Indo-European and Dravidian speaking populations in India. BMC Genet. 2014 Jul 22;15:86. DOI: 10.1186/1471-2156-15-86
[9] Agrawal AA, Kapley A, Yeltiwar RK, Purohit HJ. Assessment of single nucleotide polymorphism at IL-1A+4845 and IL-1B+3954 as genetic susceptibility test for chronic periodontitis in Maharashtrian ethnicity. J Periodontol. 2006 Sep;77(9):1515-21. DOI: 10.1902/jop.2006.050427
[10] Archana PM, Salman AA, Kumar TS, Saraswathi PK, Panishankar KH, Kumarasamy P. Association between interleukin-1 gene polymorphism and severity of chronic periodontitis in a south Indian population group. J Indian Soc Periodontol. 2012 Apr;16(2):174-8. DOI: 10.4103/0972-124X.99258
[11] Gayathri R, Saadi AV, Bhat KM, Bhat SG, Satyamoorthy K. Allele, genotype, and composite genotype effects of IL-1A +4845 and IL-1B +3954 polymorphisms for chronic periodontitis in an Indian population. Indian J Dent Res. 2011 Jul-Aug;22(4):612. DOI: 10.4103/0970-9290.90323
[12] Lavu V, Venkatesan V, Venkata Kameswara Subrahmanya Lakkakula B, Venugopal P, Paul SF, Rao SR. Polymorphic regions in the interleukin-1 gene and susceptibility to chronic periodontitis: a genetic association study. Genet Test Mol Biomarkers. 2015 Apr;19(4):175-81. DOI: 10.1089/gtmb.2014.0275
[13] Puri K, Chhokra M, Dodwad V, Puri N. Association of interleukin-1 α (-889) gene polymorphism in patients with generalized aggressive and chronic periodontitis. Dent Res J (Isfahan). 2015 Jan-Feb;12(1):76-82. DOI: 10.4103/1735-3327.150338
[14] Majumder P, Panda SK, Ghosh S, Dey SK. Interleukin gene polymorphisms in chronic periodontitis: A case-control study in the Indian population. Arch Oral Biol. 2019 May;101:156-64. DOI: 10.1016/j.archoralbio.2019.03.015
[15] Moorjani P, Thangaraj K, Patterson N, Lipson M, Loh PR, Govindaraj P, Berger B, Reich D, Singh L. Genetic evidence for recent population mixture in India. Am J Hum Genet. 2013 Sep 5;93(3):422-38. DOI: 10.1016/j.ajhg.2013.07.006
[16] Munz M, Willenborg C, Richter GM, Jockel-Schneider Y, Graetz C, Staufenbiel I, Wellmann J, Berger K, Krone B, Hoffmann P, van der Velde N, Uitterlinden AG, de Groot LCPGM, Sawalha AH, Direskeneli H, Saruhan-Direskeneli G, Guzeldemir-Akcakanat E, Keceli HG, Laudes M, Noack B, Teumer A, Holtfreter B, Kocher T, Eickholz P, Meyle J, Doerfer C, Bruckmann C, Lieb W, Franke A, Schreiber S, Nohutcu RM, Erdmann J, Loos BG, Jepsen S, Dommisch H, Schaefer AS. A genome-wide association study identifies nucleotide variants at SIGLEC5 and DEFA1A3 as risk loci for periodontitis. Hum Mol Genet. 2017 Jul 1;26(13):2577-88. DOI: 10.1093/hmg/ddx151
[17] Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgård U, Cowen EW, Pham TH, Booty M, Estes JD, Sandler NG, Plass N, Stone DL, Turner ML, Hill S, Butman JA, Schneider R, Babyn P, El-Shanti HI, Pope E, Barron K, Bing X, Laurence A, Lee CC, Chapelle D, Clarke GI, Ohson K, Nicholson M, Gadina M, Yang B, Korman BD, Gregersen PK, van Hagen PM, Hak AE, Huizing M, Rahman P, Douek DC, Remmers EF, Kastner DL, Goldbach-Mansky R. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009 Jun 4;360(23):2426-37. DOI: 10.1056/NEJMoa0807865
[18] Asensi V, Alvarez V, Valle E, Meana A, Fierer J, Coto E, Carton JA, Maradona JA, Paz J, Dieguez MA, de la Fuente B, Moreno A, Rubio S, Tuya MJ, Sarasúa J, Llames S, Arribas JM. IL-1 alpha (-889) promoter polymorphism is a risk factor for osteomyelitis. Am J Med Genet A. 2003 Jun 1;119A(2):132-6. DOI: 10.1002/ajmg.a.20137
[19] Blakemore AI, Cox A, Gonzalez AM, Maskil JK, Hughes ME, Wilson RM, Ward JD, Duff GW. Interleukin-1 receptor antagonist allele (IL1RN*2) associated with nephropathy in diabetes mellitus. Hum Genet. 1996 Mar;97(3):369-74. DOI: 10.1007/BF02185776
[20] Koss K, Satsangi J, Fanning GC, Welsh KI, Jewell DP. Cytokine (TNF alpha, LT alpha and IL-10) polymorphisms in inflammatory bowel diseases and normal controls: differential effects on production and allele frequencies. Genes Immun. 2000 Feb;1(3):185-90. DOI: 10.1038/sj.gene.6363657
[21] Li H, Groop L, Nilsson A, Weng J, Tuomi T. A combination of human leukocyte antigen DQB1*02 and the tumor necrosis factor alpha promoter G308A polymorphism predisposes to an insulin-deficient phenotype in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003 Jun;88(6):2767-74. DOI: 10.1210/jc.2002-020506
[22] Mwantembe O, Gaillard MC, Barkhuizen M, Pillay V, Berry SD, Dewar JB, Song E. Ethnic differences in allelic associations of the interleukin-1 gene cluster in South African patients with inflammatory bowel disease (IBD) and in control individuals. Immunogenetics. 2001;52(3-4):249-54. DOI: 10.1007/s002510000265
[23] Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013 Jun;62(1):59-94. DOI: 10.1111/j.1600-0757.2012.00457.x
[24] Brodzikowska A, Górski B, Bogusławska-Kapała A. Association between IL-1 Gene Polymorphisms and Stage III Grade B Periodontitis in Polish Population. Int J Environ Res Public Health. 2022 Nov 9;19(22):14687. DOI: 10.3390/ijerph192214687
[25] Vieira AR, Albandar JM. Role of genetic factors in the pathogenesis of aggressive periodontitis. Periodontol 2000. 2014 Jun;65(1):92-106. DOI: 10.1111/prd.12021
[26] Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontol 2000. 2012 Feb;58(1):37-68. DOI: 10.1111/j.1600-0757.2011.00415.x
[27] Slots J. Periodontitis: facts, fallacies and the future. Periodontol 2000. 2017 Oct;75(1):7-23. DOI: 10.1111/prd.12221




