Selection of parameters for thermal coronavirus inactivation – a data-based recommendation
Martin Hessling 1Katharina Hoenes 1
Christian Lingenfelder 2
1 Institute of Medical Engineering and Mechatronics, Ulm University of Applied Sciences, Ulm, Germany
2 Pharmpur GmbH, Koenigsbrunn, Germany
Abstract
Background: Healthcare workers and large parts of the population are currently using personal protective equipment, such as face masks, to avoid infections with the novel coronavirus SARS-CoV-2. This equipment must be sterilized as gently as possible before reuse. One possibility is thermal inactivation, but professional autoclaves with their high temperatures are often not available or suitable. If the inactivation period is long enough, coronavirus inactivation can also be carried out at relatively low temperatures. The required duration was determined in this study.
Material and methods: Data from published thermal inactivation studies on coronaviruses were applied to determine the temperature dependence of the rate constant k(T) for each coronavirus by employing Arrhenius models.
Results: The data obtained exhibit large variations, which appear to be at least partially caused by different sample properties. Samples with high protein content or samples in dry air sometimes seem to be more difficult to inactivate. Apart from this, the Arrhenius models describe the thermal inactivation properties well and SARS-CoV and SARS-CoV-2 can even be represented by a combined model. Furthermore, the available data suggest that all samples, including critical ones, can be mathematically included by a worst-case Arrhenius model.
Conclusion: Coronaviruses can already be inactivated at relatively low temperatures. For most samples, application times of approximately 32.5, 3.7, and 0.5 minutes will be sufficient at 60°C, 80°C, and 100°C, respectively, for a 5 log-reduction. For difficult conditions, the worst-case model provides significantly longer application times of 490, 55, and 8 minutes for the temperatures mentioned.
Keywords
coronavirus, SARS-CoV, SARS-CoV-2, MERS-CoV, heat, thermal inactivation, sterilization
Introduction
The emergence of the novel coronavirus SARS-CoV 2 in Wuhan (China) in 2019 has led to a pandemic that is still spreading. In order to contain its further expansion, personal protective equipment, such as face masks, is increasingly being used both in the healthcare system and among the population. This sudden rise in demand has led to a worldwide shortage, so that even disposable items are sometimes used several times [1], [2], [3]. This leads to the question of how coronaviruses in or on such disposables, but also in or on home-made or professionally manufactured fabric masks or other solid or liquid materials, including waste and human samples, can be inactivated as gently and effectively as possible?
Thermal inactivation, which in the professional sector is often carried out with steam sterilizers at temperatures of up to 121°C or even 135°C, has long been known as a very effective disinfection technique for a wide range of pathogens [4], [5], [6]. However, this approach with its typically high temperatures is not suitable for all materials and is not available for many users.
A recent overview on coronavirus inactivation temperatures by Kampf et al. [7], as well as the first corresponding investigations on SARS-CoV-2 [8], [9], [10], [11], [12], [13], [14], reveal that coronaviruses can also be inactivated by temperatures well below 120°C. Even temperatures of 60°C or lower can reduce the coronavirus load, in which the duration of the heat application increases with decreasing temperature.
In a simple first-order reaction model, the relationship between virus concentration c(t) and time t can be described by an exponential function with the rate constant k [15], [16].
Equation 1
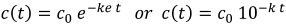
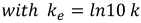
For coronaviruses, this can be observed particularly well in the experimental data of Hofmann and Wyler ([17] Fig. 2), Daniel and Talbot ([18], Fig. 2) or Laude ([19], Fig. 1), and this relationship was also applied in the coronavirus investigations of Laude [19] and Liu [20].
The rate constant k=k(T) is a function of the inactivation temperature T, which can be mathematically described by an Arrhenius approach [15], [16]. This has also successfully been applied in previous coronavirus studies [19], [20]. In short, this approach describes k(T) as an exponential function of the temperature and two virus-dependent parameters, a and b.
Equation 2
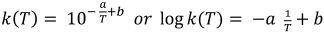
In this expression, log k(T) is represented by a straight line with the slope –a, the variable 1/T and the intercept b. The aim of this paper was to apply this mathematical model for calculating rate constants based on published coronavirus inactivation data, and use them to recommend temperature-dependent inactivation durations.
Material and methods
PubMed and Google Scholar were used to search for different combinations of the terms coronavirus, heat, temperature, inactivation, disinfection and sterilization. The references of the publications found in this process were checked for further relevant studies. Since the focus of this investigation was on heat inactivation, only data with temperatures above about 40°C and pH values between 6 and 8 were included in the further analysis, since higher and lower pH values already lead to coronavirus inactivation without additional heating [17], [18], [21], [22].
If the authors did not provide rate constants themselves, the log-reduction and the required exposure time were determined from text, tables or figures to calculate the rate constant k. If data were available for several exposure durations, as a rule, the longest exposure at which a coronavirus concentration above the detection limit was still found was chosen. The reason for this is that the heating and cooling of the investigated samples – before and after reaching the intended temperature – also contribute to inactivation, but this is not recorded separately and their relative proportions are higher for short exposures than for longer ones.
The rate constants thus obtained for different temperatures were then applied to generate equations for k(T) for the different coronaviruses according to the Arrhenius model.
Results and discussion
About 35 publications on thermal inactivation of the human viruses human coronavirus (HCoV), Middle East respiratory syndrome coronavirus (MERS-CoV), severe acute respiratory syndrome coronavirus (SARS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were found. Additionally, inactivation data for the following animal viruses was available: bovine coronavirus (BCoV), canine coronavirus (CCoV), feline infectious peritonitis coronavirus (FIPV), infectious bronchitis coronavirus (IBV), murine coronavirus (MHV), porcine epidemic diarrhea virus (PEDV), and transmissible gastroenteritis virus (TGEV). The studies contain approx. 120 data sets that met the above-mentioned selection criteria with regard to temperature and pH. However, not all of them could be evaluated quantitatively, as in some cases, only lower limits for the achieved log-reduction are given.
Attachment 1 [Att. 1] provides an overview of the obtained data, sorted by coronavirus and inactivation temperature.
The data exhibit large variability. The rate constants can differ by an order of magnitude even for one temperature and one coronavirus species. Some of these variations are probably caused by experimental inaccuracies. This can be assumed, for example, from the fact that in some experiments the determined virus concentration not only decreased but also increased again at certain phases during incubation. A further experimental factor is the effect of the heating and cooling phases already mentioned above, which can be particularly noticeable for short inactivation periods. Strain differences also contribute to this variability [19], [23], [24].
Another aspect tat has been observed in earlier publications is the influence of medium or environment. In liquid media, higher protein concentrations often seem to reduce virus inactivation [25], [26], [27] but exceptions also appear to exist [28]. Similar influences of environmental factors are observed in experiments on surfaces, which differ when performed at varying humidities, with low humidities impeding inactivation [29], [30], [31].
These large variations can also be observed in the logarithmic representation of the determined rate constants log(k), which are plotted separately for human and animal coronaviruses in Figure 1 [Fig. 1] and Figure 2 [Fig. 2] using the reciprocal of temperature 1/T (in Kelvin).
Figure 1: Logarithmic representation of the determined rate constants log k(T) for the human coronaviruses SARS-CoV, SARS-CoV-2, MERS-CoV and HCoV, together with linear regressions for SARS-CoV-2 and SARS-CoV & SARS-Co-V-2 together. A “worst-case model” was generated by shifting the SARS-CoV & SARS-CoV-2 regression curve, so that all determined rate constants for human coronaviruses are just above this line.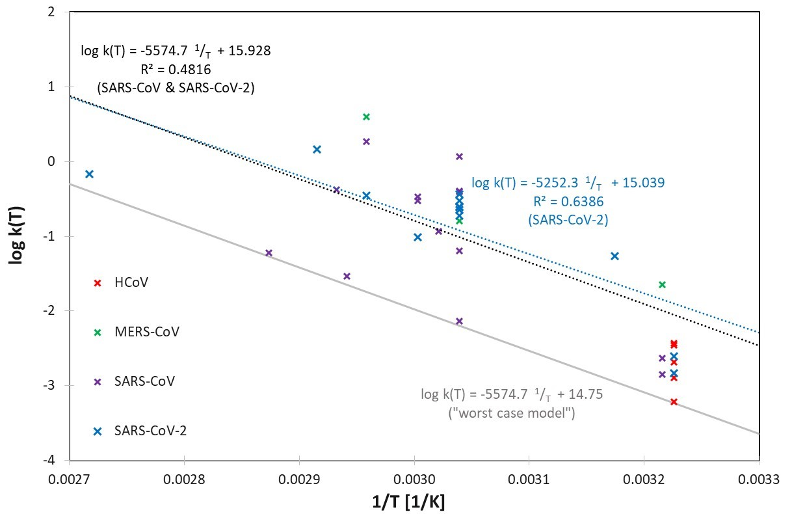
Figure 2: Logarithmic representation of the determined rate constants log k(T) for the animal coronaviruses MHV, PEDV, TGEV, IBV, BCoV, FIPV and CCoV together with a combined linear regression for log k(T) for all animal coronaviruses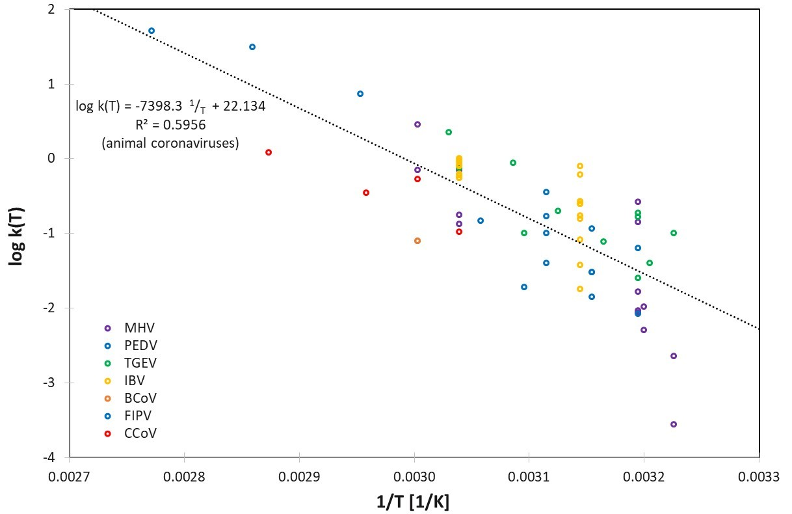
Apart from the variabilities mentioned above, and the differences for the individual coronavirus species represented in Table 1 [Tab. 1], the rate constants k(T) seem to be described relatively well by the assumed Arrhenius model. In Figure 2 [Fig. 2], even a single set of parameters appears to be suitable to describe all 7 different animal coronavirus species.
Table 1: Mathematical description of the rate constants k(T) for the different coronaviruses based on the Arrhenius model and linear regression for log k(T)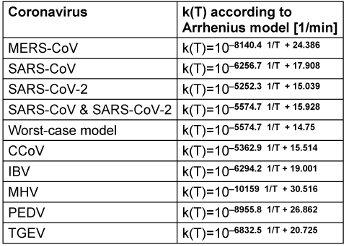
Figure 1 [Fig. 1] illustrates the behavior of the three human coronaviruses for which data on different inactivation temperatures are available. As in the analysis of animal coronaviruses, it is also consistent with the assumption of an Arrhenius model. MERS-CoV seems to be slightly more temperature sensitive, but the data of SARS-CoV and SARS-CoV-2, which belong to the same betacoronavirus subgenus sarbecovirus, exhibit similar temperature behaviors and can even be well described by a combined Arrhenius model. If the straight line belonging to this model is shifted downwards, which corresponds to an assumed reduction of the rate constant, a mathematical description for k(T) is obtained that describes a worst-case scenario, e.g., with inactivation under particularly unfavorable conditions.
With the data for k(T) from Table 1 [Tab. 1] the inactivation duration for a selected temperature T and a desired log-reduction LR can be calculated by
Equation 3
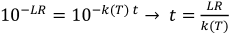
As an example of the application of these models, Table 2 [Tab. 2] provides necessary inactivation durations for a targeted 5 log-reduction for SARS-CoV or SARS-CoV-2 at different temperatures under assumed standard and worst-case conditions. (The temperature T must be entered in degrees Kelvin, which is the temperature in degrees Celsius plus 273.)
Table 2: Necessary inactivation durations for thermal SARS-CoV or SARS CoV-2 inactivation for standard and worst-case conditions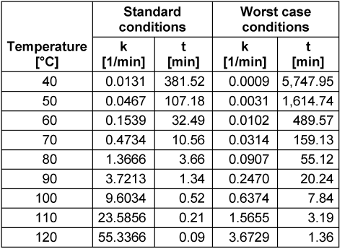
Conclusions
All investigations prove that coronaviruses can be inactivated quite quickly at relatively low temperatures, e.g., 60°C or 80°C. These are temperatures for which no professional autoclaves are necessary, but which can even be achieved with domestic cooking equipment such as an oven or rice cooker.
The observed temperature dependence of coronavirus inactivation behavior – represented by the rate constant k(T) – is well described by Arrhenius models, so that necessary inactivation times can be calculated for each coronavirus and each inactivation temperature.
The two particularly important and related human coronaviruses SARS-CoV and SARS-CoV-2 behave very similarly and can be described by a combined Arrhenius model. This model delivers inactivation durations of approx. 32.5, 3.7 and 0.5 minutes for temperatures of 60°C, 80°C and 100°C, respectively, for a 5 log-reduction. For potentially critical samples the proposed worst-case model should be applied for safety reasons.
Notes
Competing interests
The authors declare that they have no competing interests.
References
[1] Mahase E. Novel coronavirus: Australian GPs raise concerns about shortage of face masks. BMJ. 2020 Feb;368:m477. DOI: 10.1136/bmj.m477[2] Rowan NJ, Laffey JG. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic – Case study from the Republic of Ireland. Sci Total Environ. 2020 Jul;725:138532. DOI: 10.1016/j.scitotenv.2020.138532
[3] Chughtai AA, Seale H, Islam MS, Owais M, Macintyre CR. Policies on the use of respiratory protection for hospital health workers to protect from coronavirus disease (COVID-19). Int J Nurs Stud. 2020 May;105:103567. DOI: 10.1016/j.ijnurstu.2020.103567
[4] Smelt JP, Brul S. Thermal inactivation of microorganisms. Crit Rev Food Sci Nutr. 2014;54(10):1371-85. DOI: 10.1080/10408398.2011.637645
[5] Rahn O. Physical methods of sterilization of microorganisms. Bacteriol Rev. 1945 Mar;9(1):1-47.
[6] Chmiel H, Takors R, Weuster-Botz D, editors. Bioprozesstechnik. 4th ed. Berlin, Heidelberg: Springer Spektrum; 2018. DOI: 10.1007/978-3-662-54042-8
[7] Kampf G, Voss A, Scheithauer S. Inactivation of coronaviruses by heat. J Hosp Infect. 2020 Jun;105(2):348-9. DOI: 10.1016/j.jhin.2020.03.025
[8] Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen HL, Chan MCW, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1):e10. DOI: 10.1016/S2666-5247(20)30003-3
[9] Wang T, Lien C, Liu S, Selveraj P. Effective Heat Inactivation of SARS-CoV-2 [Preprint]. medRxiv. 2020 May 5. DOI: 10.1101/2020.04.29.20085498
[10] Pastorino B, Touret F, Gilles M, Lamballerie X de, Charrel RN. Evaluation of heating and chemical protocols for inactivating SARS-CoV-2 [Preprint]. bioRxiv. 2020 Apr 20. DOI: 10.1101/2020.04.11.036855
[11] Batéjat C, Grassin Q, Manuguerra JC, Leclercq I. Heat inactivation of the Severe Acute Respiratory Syndrome Coronavirus 2 [Preprint]. bioRxiv. 2020 May 1. DOI: 10.1101/2020.05.01.067769
[12] Jureka AS, Silvas JA, Basler CF. Propagation, inactivation, and safety testing of SARS-CoV-2 [Preprint]. bioRxiv. 2020 May 14. DOI: 10.1101/2020.05.13.094482
[13] Pitino MA, O'Connor DL, McGeer AJ, Unger S. The impact of thermal pasteurization on viral load in human milk and other matrices: A rapid review [Preprint]. medRxiv. 2020 May 26. DOI: 10.1101/2020.05.23.20111369
[14] Patterson EI, Prince T, Anderson ER, Casas-Sanchez A, Smith SL, Cansado-Utrilla C, et al. Methods of inactivation of SARS-CoV-2 for downstream biological assays [Preprint]. bioRxiv. 2020 May 23. DOI: 10.1101/2020.05.21.108035
[15] Hiatt CW. Kinetics of the inactivation of viruses. Bacteriol Rev. 1964 Jun;28:150-63. DOI: 10.1128/MMBR.28.2.150-163.1964
[16] Gard S, Maaloe O. Inactivation of Viruses. In: Burnet FM, editor. General virology: Biochemical, biological and biophysical properties. London: Academic Press; 2013. p. 359-427. DOI: 10.1016/B978-1-4832-2765-8.50011-7.
[17] Hofmann M, Wyler R. Quantitation, biological and physicochemical properties of cell culture-adapted porcine epidemic diarrhea coronavirus (PEDV). Vet Microbiol. 1989 Jun;20(2):131-42. DOI: 10.1016/0378-1135(89)90036-9
[18] Daniel C, Talbot PJ. Physico-chemical properties of murine hepatitis virus, strain A 59. Brief report. Arch Virol. 1987;96(3-4):241-8. DOI: 10.1007/BF01320963
[19] Laude H. Thermal inactivation studies of a coronavirus, transmissible gastroenteritis virus. J Gen Virol. 1981 Oct;56(Pt 2):235-40. DOI: 10.1099/0022-1317-56-2-235
[20] Liu J. A rate equation approach to model the denaturation or replication behavior of the SARS coronavirus. Forsch Ingenieurwes. 2004;68(4):227-38. DOI: 10.1007/s10010-004-0130-2
[21] Lamarre A, Talbot PJ. Effect of pH and temperature on the infectivity of human coronavirus 229E. Can J Microbiol. 1989 Oct;35(10):972-4. DOI: 10.1139/m89-160
[22] Darnell ME, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Methods. 2004 Oct;121(1):85-91. DOI: 10.1016/j.jviromet.2004.06.006
[23] Otsuki K, Noro K, Yamamoto H, Tsubokura M. Studies on avian infectious bronchitis virus (IBV). II. Propagation of IBV in several cultured cells. Arch Virol. 1979;60(2):115-22. DOI: 10.1007/BF01348027
[24] Luytjes W, Gerritsma H, Bos E, Spaan W. Characterization of two temperature-sensitive mutants of coronavirus mouse hepatitis virus strain A59 with maturation defects in the spike protein. J Virol. 1997 Feb;71(2):949-55.
[25] Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005 Jan;194(1-2):1-6. DOI: 10.1007/s00430-004-0219-0
[26] Darnell ME, Taylor DR. Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion. 2006 Oct;46(10):1770-7. DOI: 10.1111/j.1537-2995.2006.00976.x
[27] Yunoki M, Urayama T, Yamamoto I, Abe S, Ikuta K. Heat sensitivity of a SARS-associated coronavirus introduced into plasma products. Vox Sang. 2004 Nov;87(4):302-3. DOI: 10.1111/j.1423-0410.2004.00577.x
[28] Quist-Rybachuk GV, Nauwynck HJ, Kalmar ID. Sensitivity of porcine epidemic diarrhea virus (PEDV) to pH and heat treatment in the presence or absence of porcine plasma. Vet Microbiol. 2015 Dec;181(3-4):283-8. DOI: 10.1016/j.vetmic.2015.10.010
[29] Casanova LM, Jeon S, Rutala WA, Weber DJ, Sobsey MD. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl Environ Microbiol. 2010 May;76(9):2712-7. DOI: 10.1128/AEM.02291-09
[30] Chan KH, Peiris JS, Lam SY, Poon LL, Yuen KY, Seto WH. The Effects of Temperature and Relative Humidity on the Viability of the SARS Coronavirus. Adv Virol. 2011;2011:734690. DOI: 10.1155/2011/734690
[31] Li DF, Cadnum JL, Redmond SN, Jones LD, Donskey CJ. It's not the heat, it's the humidity – Effectiveness of a rice cooker-steamer for decontamination of cloth and surgical face masks and N95 respirators. Am J Infect Control. 2020;S0196-6553(20)30238-8. DOI: 10.1016/j.ajic.2020.04.012
[32] Akashi H, Inaba Y, Miura Y, Tokuhisa S, Sato K, Satoda K. Properties of a coronavirus isolated from a cow with epizootic diarrhea. Vet Microbiol. 1980;5:265-76. DOI: 10.1016/0378-1135(80)90025-5.
[33] Panon G, Tache S, Labie C. Respective stability of rotavirus and coronavirus in bovine milk. Lait. 1988;68:49-64. DOI: 10.1051/lait:198814
[34] Pratelli A. Canine coronavirus inactivation with physical and chemical agents. Vet J. 2008 Jul;177(1):71-9. DOI: 10.1016/j.tvjl.2007.03.019
[35] Saknimit M, Inatsuki I, Sugiyama Y, Yagami K. Virucidal efficacy of physico-chemical treatments against coronaviruses and parvoviruses of laboratory animals. Jikken Dobutsu. 1988 Jul;37(3):341-5. DOI: 10.1538/expanim1978.37.3_341
[36] Christianson KK, Ingersoll JD, Landon RM, Pfeiffer NE, Gerber JD. Characterization of a temperature sensitive feline infectious peritonitis coronavirus. Arch Virol. 1989;109(3-4):185-96. DOI: 10.1007/BF01311080
[37] Bucknall RA, King LM, Kapikian AZ, Chanock RM. Studies with human coronaviruses. II. Some properties of strains 229E and OC43. Proc Soc Exp Biol Med. 1972 Mar;139(3):722-7. DOI: 10.3181/00379727-139-36224
[38] Leclercq I, Batéjat C, Burguière AM, Manuguerra JC. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir Viruses. 2014 Sep;8(5):585-6. DOI: 10.1111/irv.12261
[39] Hirano N, Hino S, Fujiwara K. Physico-chemical properties of mouse hepatitis virus (MHV-2) grown on DBT cell culture. Microbiol Immunol. 1978;22(7):377-90. DOI: 10.1111/j.1348-0421.1978.tb00384.x
[40] Cheever FS, Daniels JB. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. J Exp Med. 1949 Sep;90(3):181-210. DOI: 10.1084/jem.90.3.181
[41] Lelie PN, Reesink HW, Lucas CJ. Inactivation of 12 viruses by heating steps applied during manufacture of a hepatitis B vaccine. J Med Virol. 1987 Nov;23(3):297-301. DOI: 10.1002/jmv.1890230313
[42] Hulst MM, Heres L, Hakze-van der Honing RW, Pelser M, Fox M, van der Poel WHM. Study on inactivation of porcine epidemic diarrhoea virus, porcine sapelovirus 1 and adenovirus in the production and storage of laboratory spray-dried porcine plasma. J Appl Microbiol. 2019 Jun;126(6):1931-43. DOI: 10.1111/jam.14235
[43] Zentkovich MM, Nelson SW, Stull JW, Nolting JM, Bowman AS. Inactivation of porcine epidemic diarrhea virus using heated water. Vet Anim Sci. 2016 Dec;1:1-3. DOI: 10.1016/j.vas.2016.09.001
[44] Pujols J, Segalés J. Survivability of porcine epidemic diarrhea virus (PEDV) in bovine plasma submitted to spray drying processing and held at different time by temperature storage conditions. Vet Microbiol. 2014 Dec;174(3-4):427-32. DOI: 10.1016/j.vetmic.2014.10.021
[45] Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Jpn J Vet Res. 2004 Nov;52(3):105-12.
[46] Duan SM, Zhao XS, Wen RF, Huang JJ, Pi GH, Zhang SX, Han J, Bi SL, Ruan L, Dong XP; SARS Research Team. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci. 2003 Sep;16(3):246-55.
[47] Pagat AM, Seux-Goepfert R, Lutsch C, Lecouturier V, Saluzzo JF, Kusters IC. Evaluation of SARS-Coronavirus Decontamination Procedures. Appl Biosaf. 2007;12(2):100-8. DOI: 10.1177/153567600701200206
[48] Kumar A, Kasloff SB, Leung A, Cutts T, Strong JE, Hills K, Vazquez-Grande G, Rush B, Lother S, Zarychanski R, Krishnan Jl. N95 Mask Decontamination using Standard Hospital Sterilization Technologies [Preprint]. medRxiv. 2020 Apr 20. DOI: 10.1101/2020.04.05.20049346
[49] Cartwright SF. A cytopathic virus causing a transmissible gastroenteritis in swine. II. Biological and serological studies. J Comp Pathol. 1966 Jan;76(1):95-106. DOI: 10.1016/0021-9975(66)90051-x
Attachments
| Attachment 1 | Attachment 1: Coronavirus inactivation data by coronavirus and temperature (Attachment 1.pdf, application/pdf, 151.02 KBytes) |




