Anatomy-based CI fitting via preoperative imaging (preopABF)
Daniel Polterauer 1,2Carmen Molenda 1,2
Andrea Schreier 1,2
Sarah Draut 1,2
Maike Neuling 1,2
John Martin Hempel 1,2
Joachim Müller 1,2
1 Department and Polyclinic of Otorhinolaryngology, University Hospital of Munich, LMU Munich, Germany
2 Section of Otology and Cochlear Implants, University Hospital of Munich, LMU Munich, Germany
Abstract
The use of software for measuring the inner ear anatomy allows for the estimation of the position of the electrode array contacts in the cochlea prior to cochlear implantation, based on the standard preoperative imaging. These estimated contact positions can be used in the cochlear implant (CI) fitting software instead of the standard frequency table (“preopABF”). In initial and follow-up fittings, the preference for preopABF vs. standard fitting was investigated. The electrode positions calculated from preoperatively available CTs and MRIs from clinical routine were recorded using the software OTOPLAN4 (CAScination, Bern, Switzerland; distributed by MED-EL, Innsbruck, Austria). In both initial and follow-up fittings, preopABF and standard fitting were offered in a single-blind manner. If there was a clear subjective preference, only that variant was programmed into the speech processor. In all other cases, both variants were programmed as programs. PreopABF was activated for 47 patients. Of these 47 patients, 6 patients (each bilaterally CI-supplied) declined the experimental comparison of standard frequency allocation and that provided by preopABF (=ipsilateral ear). However, 5 of these 6 patients preferred preopABF on the contralateral ear, regardless of the delay between 1st ear and 2nd ear cochlear implantation. Only one patient rejected preopABF bilaterally and was therefore excluded from further analysis. The remaining 46 patients consented to a comparison between the traditional fitting and the novel preopABF (at least on one ear). In two cases, bilateral preopABF was performed, and in 44 cases, unilateral preopABF was performed. The estimated electrode positions obtained from preoperative CT or MRI using OTOPLAN were successfully used in clinical practice for CI fitting (preopABF) and showed a clear preference over the standard setting in this evaluation. Whether a CI fitting based on postoperative imaging (preopABF) will show a significant patient preference in comparison to standard fitting is still to be investigated.
Keywords
anatomy-based CI fitting, preopABF, tonotopic mismatch
Introduction
Cochlear implantation (CI) has now become a standard therapy option for severe sensorineural hearing loss. Nevertheless, post-implantation speech understanding remains highly variable and is therefore a central focus of current research [1].
Significant correlations with speech understanding have been shown for many influencing factors, including the so-called tonotopic “mismatch”. This refers to the discrepancy between the region stimulated by the implant at a certain frequency in the cochlea and the location that would be stimulated at that frequency in a normal cochlea, resulting in a mislocalization of a frequency in the tonotopic organization of the cochlea (and the subsequent auditory pathway). This is especially relevant for post-lingually deafened patients who have already learned the relationship between frequency and tonotopic place [2] and can lead to poorer speech understanding [3]. Therefore, different strategies have been recently explored to reduce the tonotopic “mismatch” (electrode type, electrode insertion depth, electrode positions, surgical approach (round window vs. cochleostomy), etc.) [4], [5].
Recently, in this context, anatomy-based fitting (ABF) was also introduced [6]. Here, a new image-based surgical planning software displays the exact electrode position after postoperative computed tomography, allowing for individual, anatomy-based fitting. It has been demonstrated that ABF can lead to improved speech understanding. However, unlike preoperative imaging, postoperative imaging using CT is not part of common clinical practice and in some cochlear implant clinics even no postoperative imaging at all. With the surgical planning software provided by the manufacturer MED-EL (Innsbruck, Austria), the expected electrode positions can be estimated preoperatively depending on the selected electrode array (FLEX, FORM, or CLASSIC series) from the preoperative imaging, serving as the basis for ABF (“preopABF”).
The aim of this study was to investigate the preference for preopABF vs. standard fitting in initial and follow-up fittings.
Methods
As part of the preoperative analysis of the imaging data, either the available CT or MRI was selected per ear based on image quality and slice thickness [7]. This analysis was performed using OTOPLAN4 (CAScination, Bern, Switzerland; distributed by MED-EL, Innsbruck, Austria). In older versions up to and including OTOPLAN3, the analysis of the inner ear had to be done manually [8]. In case of already given OTOPLAN3 analyses, these where re-checked via OTOPLAN4 in the setting reported below.
With OTOPLAN4, it is now possible to automatically analyze imaging data, provided a pixel spacing of ≤0.5 mm, a slice thickness of ≤0.6 mm, and a slice gap of ≤0.6 mm are present. Primarily, the CT was evaluated, as the requirements for automatic evaluation are typically more frequently met here. If automatic evaluation was not possible, the CT or MRI, depending on which had the better images, was manually evaluated. In the case of manual evaluation, this was always carried out by an experienced OTOPLAN user. Only imaging data with a slice thickness of ≤1.0 mm were evaluated. To ensure accurate evaluation, the OTOPLAN measurement was performed on a large monitor with high resolution, high brightness, and high-quality color reproduction (HUAWEI MateView GT 34”).
The measured parameters were then used to plan the electrode array selection. Typically, planning was done with a fully inserted FLEXSOFT electrode array. If a coverage ≥85% was achieved, a shorter electrode array could be planned. If coverage with FLEXSOFT was ≤85%, planning was done with a FLEX34, which represents the currently longest electrode array [9]. In addition to a report (containing data on cochlear parameters, electrode insertion angles and other information), OTOPLAN4 generates an XML file containing the frequency allocation of the band pass filter settings of the speech processor map for individual stimulation contacts of the electrode array. In cases where the scheduled electrode array was unexpectedly not used during the surgery, the actually implanted electrode array was entered in OTOPLAN, and a new export of the report and XML file was generated.
If a patient was already implanted, a retrospective approach based on the preoperative imaging data was followed using the same principles as described in the scenario above. The resulting XML files with frequency allocations based on the preoperative imaging data on the implanted electrode array were stored in the fitting software MAESTRO (MED-EL), allowing for the option of preopABF. The data was used as given by MAESTRO and not manually adjusted. A tonotopic mismatch occurs for >8,500 Hz by manufacturer’s limitation of 8,500 Hz as the maximum addressable frequency in MAESTRO.
As the basis of the fitting, the Greenwood function was selected with an angle of 990° from the round window to the helicotrema [4], [10], [11].
To compare preopABF to classical CI fitting, we ask patients at the initial preopABF fitting for testing it. In this study, the initial preopABF fitting could be first CI fitting but also any other. If there was a clear subjective preference, only that variant was programmed into the speech processor. In all other cases, both variants were programmed and stored in the patient’s processor. At the next regular appointment with a minimum of a month delay, the preopABF was checked asking the patients for their subjective preference pro or contra preopABF.
Results
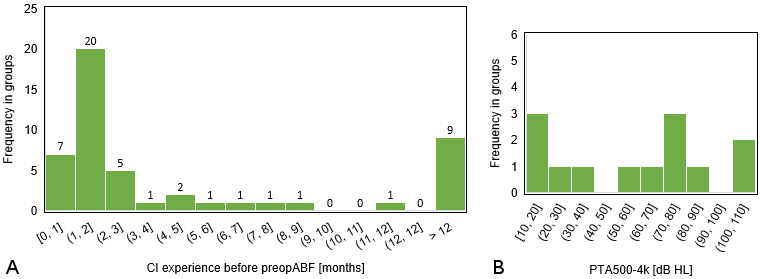
In 47 cases, such an XML file was stored and preopABF was activated. Since part of the patient collective was already adjusted (N=40), the switch to preopABF was not made during the initial fitting but sometimes later (see Figure 1 [Fig. 1]), especially when there were requests for changes in the CI settings that seemed potentially solvable by a different frequency allocation of the stimulation electrodes like unclear tone in general or regarding specific noises or voices.
During the on-site testing of preopABF in the clinical CI fitting room, either the standard frequency allocation or the preABF was randomly activated first after a standard fitting process in live mode, i.e., when the CI system was activated and the environment was captured via the microphone of the speech processor. Subsequently, the remaining frequency allocation was selected and presented to the patient as an alternative. The patient was not informed which variant included which frequency allocation.
Out of these 47 patients (see Table 1 [Tab. 1] and Table 2 [Tab. 2]), 6 patients declined the experimental comparison between standard frequency allocation and preopABF. All of these 6 patients were bilaterally implanted with a cochlear implant, but only one patient rejected preopABF bilaterally, while the other patients preferred preopABF on the secondly implanted ear, regardless of the delay between the surgeries of both ears. Therefore, only that one patient was excluded from further analysis. The remaining 46 patients agreed to a comparison between the traditional fitting and the novel preopABF (at least on one ear). Among them, bilateral preopABF was performed in two cases, and unilateral preopABF in 44 cases (5/44 patients with bilateral CI provision but rejecting preopABF on the opposite side as mentioned above, 29/44 patients with bimodal provision, 7/44 patients without a hearing aid indication on the contralateral side (i.e., single-sided-deafness), 3/44 patients with an indication for but without provision).
Having a closer look at the bilateral CI, these patients showed a similar pattern. All bilateral CI patients told us that the test program using preopABF was not different to their old one and therefore they preferred to stay with the standard programming. Patients in this group had a long time of CI experience at least in one ear. This seems to lead to a low acceptance for changes in general regarding CI fitting and seems to be true also for the preopABF. On the other hand, unilateral CI patients seem to be much more likely to accept preopABF in our patient group.
![]()
Equation 1: Calculation of the electrical cochlear coverage (=eCC) using the C1 place in mm and the full OC CDL in mm. By this, we respect the hook region (2.5 mm) in contrast to, e.g., calculations of the mechanical cochlear coverage.
Discussion
The aim of the study was to investigate whether in initial and follow-up fittings an individual, anatomy-based adjustment based on preoperative imaging and using a new, image-based surgical planning software (preopABF) can achieve a higher preference compared to standard adjustments. The hypothesis is that such a fitting tailored to individual anatomy reduces tonotopic mismatch and can lead to better speech understanding post-implantation, which seems to be the case with anatomy-based adjustments based on postoperative imaging according to current research [12], [13], [14], [15], [16]. Sturm et al. showed that with a lower mismatch, there was comparable speech understanding but higher quality of life [17].
The estimated electrode positions obtained from preoperative CT or MRI using OTOPLAN could be used for CI fitting in clinical practice with high patient preference. In this analysis, patients showed a clear preference for preopABF over the posistandard setting. 46 out of 47 patients, i.e., 98% of patients, not only tested preopABF but also kept it as the standard setting in at least one CI system after the test (in the case of bilateral CI provision).
Only 6 out of 47 patients rejected preopABF altogether. These patients were all bilaterally implanted with a cochlear implant. Only one patient rejected preopABF bilaterally. Interestingly, 5 out of these 6 patients preferred preopABF on the contralateral side without showing a tendency towards whether preopABF was accepted more for the first or second implanted ear. Thus, it seems that even the patient group with bilateral CI provision can benefit from preopABF, as shown by postoperative ABF in the study by Kurz et al. [6].
One limitation of the study is the small number of cases. However, this is an initial pilot study, and given the high preference for preopABF by patients, a larger number of patients will be recruited in the future. Another limitation is that preopABF may not be as precise as ABF based on postoperative imaging. However, this hypothesis needs appropriate studies for clarification. PreopABF is a simple, practical, and pragmatic solution that avoids additional radiation exposure raised by postoperative imaging, which is also costly and not widely available. Future studies should address naive patients without CI experience. This way, the usefulness of preopABF in bilaterally implanted patients can be analyzed.
An interesting alternative approach, especially in children, is the use of a post-CI surgery X-ray, which is routine in most clinics and causes significantly lower radiation exposure than a postoperative CT scan [18]. This will be available in the upcoming OTOPLAN5.
Lastly, it is important to emphasize that speech understanding after CI provision is influenced by multiple factors, making it necessary to demonstrate weak effects on speech understanding through studies of the highest evidence class. The findings thus confirm the consideration of making individual, anatomy-based adjustments.
The extent to which differences can be demonstrated related to CI fitting on the basis of postoperative imaging (=postopABF) remains to be investigated. Using postopABF showed better speech understanding in noise with equal speech understanding in quiet and a strong subjective preference for the ABF setting compared to standard fitting (assuming a sufficiently long adaptation phase) [2], [4], [16], [19].
In the future, it will be intriguing to investigate CI configurations based on preopABF regarding boundary frequencies variation in the primary speech region. Creff et al. already showed a notably significant difference between postopABF and standard fitting in the primary speech region. Interestingly, they also found a reduced variability for postopABF in their study group and an improved speech comprehension [16].
Notes
Conference presentation
This contribution was presented at the 26th Annual Conference of the German Society of Audiology and published as an abstract [20].
Competing interests
The authors declare that they have no competing interests.
References
[1] Müller J, Molenda C, Polterauer D. Aktuelle Trends und Entwicklungen bei der Cochlea-Implantat-Versorgung [Current Trends and Developments in Cochlear Implantation]. Sprache Stimme Gehör. 2024;48:22-31. DOI: 10.1055/a-2195-4886[2] Svirsky MA, Talavage TM, Sinha S, Neuburger H, Azadpour M. Gradual adaptation to auditory frequency mismatch. Hear Res. 2015 Apr;322:163-70. DOI: 10.1016/j.heares.2014
[3] Canfarotta MW, Dillon MT, Buss E, Pillsbury HC, Brown KD, O'Connell BP. Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear. 2020 Sep/Oct;41(5):1349-61. DOI: 10.1097/AUD.0000000000000864
[4] Mertens G, Van de Heyning P, Vanderveken O, Topsakal V, Van Rompaey V. The smaller the frequency-to-place mismatch the better the hearing outcomes in cochlear implant recipients? Eur Arch Otorhinolaryngol. 2022 Apr;279(4):1875-83. DOI: 10.1007/s00405-021-06899-y
[5] Ali H, Noble JH, Gifford RH, Labadie RF, Dawant BM, Hansen JH, Tobey E. Image-guided customization of frequency-place mapping in cochlear implants. In: International Conference on Acoustics, Speech, and Signal Processing (ICASSP); 2015 Apr 19-24; Brisbane, Australia.
[6] Kurz A, Herrmann D, Hagen R, Rak K. Using Anatomy-Based Fitting to Reduce Frequency-to-Place Mismatch in Experienced Bilateral Cochlear Implant Users: A Promising Concept. J Pers Med. 2023 Jul 8;13(7):1109. DOI: 10.3390/jpm13071109
[7] Weber L, Kwok P, Picou EM, Wendl C, Bohr C, Marcrum SC. Vermessung der Cochlea mittels eines Tablet-basierten Softwarepakets: Einfluss der Bildgebungsmodalität und des Untersucherhintergrunds. HNO. 2022 Oct;70(10):769-77. DOI: 10.1007/s00106-022-01208-3
[8] Spiegel JL, Polterauer D, Hempel JM, Canis M, Spiro JE, Müller J. Variation of the cochlear anatomy and cochlea duct length: analysis with a new tablet-based software. Eur Arch Otorhinolaryngol. 2022 Apr;279(4):1851-61. DOI: 10.1007/s00405-021-06889-0
[9] Schreier A, Molenda C, Draut S, Hempel J, Volgger V, Polterauer D, Merz L, Müller J. Individualized cochlear implantation – a new, longer electrode array to meet the need for patients with very long cochleae: first experience with a new 34 mm electrode. In: 14th Asia Pacific Symposium on Cochlear Implant and Related Sciences (APSCI 2023); 2023 Nov 8-11; Seoul, South Korea.
[10] Greenwood DD. A cochlear frequency-position function for several species – 29 years later. J Acoust Soc Am. 1990 Jun;87(6):2592-605. DOI: 10.1121/1.399052
[11] Dillon MT, Canfarotta MW, Buss E, O'Connell BP. Comparison of Speech Recognition With an Organ of Corti Versus Spiral Ganglion Frequency-to-Place Function in Place-Based Mapping of Cochlear Implant and Electric-Acoustic Stimulation Devices. Otol Neurotol. 2021 Jun 1;42(5):721-5. DOI: 10.1097/MAO.0000000000003070
[12] Dessard L, Gersdorff G, Ivanovik N, Zoca-Assadi M, Nopp P, Camby S, Lefebvre PP. Cochlear Implant: Analysis of the Frequency-to-Place Mismatch with the Table-Based Software OTOPLAN® and Its Influence on Hearing Performance. Audiol Neurootol. 2024;29(3):239-45. DOI: 10.1159/000535693
[13] Lassaletta L, Calvino M, Sanchez-Cuadrado I, Gavilán J. Does it make any sense to fit cochlear implants according to the anatomy-based fitting? Our experience with the first series of patients. Front Audiol Otol. 2023;1:1298538. DOI: 10.3389/fauot.2023.1298538
[14] Di Maro F, Carner M, Sacchetto A, Soloperto D, Marchioni D. Frequency reallocation based on cochlear place frequencies in cochlear implants: a pilot study. Eur Arch Otorhinolaryngol. 2022 Oct;279(10):4719-25. DOI: 10.1007/s00405-021-07245-y
[15] Adunka OF, Buchman CA, Adunka OF, editors. Otology, Neurotology, and Lateral Skull Base Surgery. Thieme Verlag; 2011. DOI: 10.1055/b-002-85469
[16] Creff G, Lambert C, Coudert P, Pean V, Laurent S, Godey B. Comparison of Tonotopic and Default Frequency Fitting for Speech Understanding in Noise in New Cochlear Implantees: A Prospective, Randomized, Double-Blind, Cross-Over Study. Ear Hear. 2024 Jan-Feb 01;45(1):35-52. DOI: 10.1097/AUD.0000000000001423
[17] Sturm JJ, Ma C, McRackan TR, Schvartz-Leyzac KC. Frequency-to-Place Mismatch Impacts Cochlear Implant Quality of Life, But Not Speech Recognition. Laryngoscope. 2024 Jun;134(6):2898-905. DOI: 10.1002/lary.31264
[18] Alahmadi A, Abdelsamad Y, Thabet EM, Hafez A, Alghamdi F, Badr KM, Alghamdi S, Hagr A. Advancing Cochlear Implant Programming: X-ray Guided Anatomy-Based Fitting. Otol Neurotol. 2024 Feb 01;45(2):107-13. DOI: 10.1097/MAO.0000000000004069
[19] Jiam NT, Gilbert M, Cooke D, Jiradejvong P, Barrett K, Caldwell M, Limb CJ. Association Between Flat-Panel Computed Tomographic Imaging-Guided Place-Pitch Mapping and Speech and Pitch Perception in Cochlear Implant Users. JAMA Otolaryngol Head Neck Surg. 2019 Feb 1;145(2):109-16. DOI: 10.1001/jamaoto.2018.3096
[20] Polterauer D, Molenda C, Schreier A, Draut S, Neuling M, Hempel JM, Müller J. Anatomiebasierte CI-Anpassung via präoperativer Bildgebung (preopABF). In: Deutsche Gesellschaft für Audiologie e.V., editor. 26. Jahrestagung der Deutschen Gesellschaft für Audiologie. Aalen, 06.-08.03.2024. Düsseldorf: German Medical Science GMS Publishing House; 2024. Doc140. DOI: 10.3205/24dga140




