[Antimikrobielle Hemmwirkung wässriger, hydroalkoholischer und alkoholischer Extrakte von Blättern und Stamm von Daphne mucronata gegenüber oralen Bakterien]
Kolsoom Shirzadi Karamolah 1Fatemeh Mousavi 1
Hassan Mahmoudi 2
1 Islamic Azad University, Boroujerd Branch, Lorstan, Iran
2 Microbiology Department, Hamadan University of Medical Sciences, Hamadān, Iran
Zusammenfassung
Zielsetzung: Pflanzen sind Quelle potentiell antimikrobiell wirksamer Verbindungen. Zielsetzung der vorliegenden Studie war die Bestimmung der antimikrobiellen Hemmkonzentration wässriger, hydroalkoholischer und alkoholischer Extrakte aus Blättern und Stamm von Daphne mucronata gegenüber oralen Bakterien.
Material und Methode: Blätter und Stamm von Daphne mucronata wurden im Zagrosgebirge in der Provinz Lorestan, Iran, gesammelt. Sie wurden im Schatten getrocknet. Die Extrakte wurden mittels klassischer Technik zur Lösungsmittelextraktion aus Pflanzen gewonnen. Die antimikrobielle Wirkung wurde im Agarplättchendiffusionstest und als minimale Hemmkonzentration ermittelt. Die Ergebnisse wurden mittels Duncan's und ANOVA Test analysiert.
Ergebnisse: Die antimikrobielle Wirksamkeit war abhängig von der Art der Extraktion. Der alkoholische Extrakt von Daphne mucronata war am wirksamsten mit der höchsten Effektivität gegenüber Streptococcus mutans, während der wässrige Extrakt unwirksam war.
Schlussfolgerung: Aufgrund der Ergebnisse könnte der alkoholische Extrakt von Daphne mucronata ein geeigneter Kandidat im Rahmen der Erforschung natürlicher antimikrobieller Wirkstoffe und speziell zur Prävention von Streptococcus mutans aussichtsreich sein.
Schlüsselwörter
Daphne mucronata, antimikrobielle Hermmwirkung, Agarplättchendiffusionstest, minimale Hemmkonzentration
Introduction
Bacterial resistance to antibiotics has become a global problem. In recent years, much attention has been focused on the use of herbal medicine, due to fewer side effects [1]. The problem of antibiotic resistance is based on many factors, e.g., inappropriate use of antibiotics [2]. Owing to the beneficial properties of medicinal plants in the treatment of diseases and with regard to the availability and compatibility with the human immune system, their use is on the rise [3], [4], [5]. Daphne mucronata is a wild shrub of the family Thymelaeaceae, which is found in many parts of Iran [6]. This plant has been traditionally used to treat skin diseases and cancer. The daphnetin 8 glycoside obtained from D. mucronata might have cardiotoxic effects [7], [8], [9]. Cytotoxic activity of a hydroalcoholic extract of D. mucronata on different cells has been reported previously, mostly on lung cancer cells [10], [11], [12], [13]. D. mucronata is used in the northern areas of Pakistan for patients suffering from hot flashes, arthritis, fever, and muscle pain, as well as for topical treatment of inflammation and to treat gastric ulcers, rheumatism, dental decay and toothache [14], [15]. Studies have shown that Daphne spp. are a potential source of anti-biofilm agents [16], [17]. Daphne gnidium grows in Mediterranean regions, and extracts of its leaves and bark have been shown to possess antimicrobial and germicidal activity [11]. Therefore, this study aimed to investigate the therapeutic effects of Daphne mucronata as an alternative to synthetic drugs and chemical mouthwashes.
Methods
Collection of plant materials
The leaves and stems of Daphne mucronata were collected in the spring and summer (April–July 2015) from the Zagros Mountains, Lorestan, Iran and were authenticated by Department of Pharmacognosy and Pharmaceutical Biotechnology, School of Pharmacy, Hamadan University of Medical Science, Hamadan, Iran.
Extraction
Aqueous, hydroalcoholic and alcoholic extracts of the plant were prepared by soaking the dried leaves and stems using the percolation method.
For the aqueous extract, 10 g of leaf powder were soaked in 100 ml of boiled, distilled water for 2 h at 60–70°C. The decoction was filtered and evaporated in a water bath at 50–60°C to yield a solid extract.
The ethanol extract was prepared using the same protocol. Dried, powdered plant materials were subjected to extraction using 95% ethanol and hydroalcohol (50% ethanol) for 6 h at 37°C. The extraction procedure was repeated thrice. The combined ethanolic and hydroalcoholic extracts were pooled and evaporated dry under reduced pressure at 40°C with rotator vacuum evaporator. Therefore, residual alcohol cannot affect the results. The percentage yields of the crude extracts were found to be 14.98% w/w for 95% ethanolic and 15.58% w/w for hydroalcoholic extracts.
Concentrations of 6.25, 12.5, 25, 50, and 100 mg/ml of the leaf and stem extract solution were dissolved in dimethyl sulfoxide (DMSO), and 30 µl of each concentration was inoculated on to a blank disk [18]. Next the disks were kept at 25 °C for 5 hours for drying.
Test organisms
In order to evaluate the antimicrobial properties, the gram-positive bacteria Staphylococcus epidermidis (ATCC12228), S. aureus (ATCC 10690) and Streptococcus mutans (ATCC 10672) and the gram negative bacteria Neisseria sicca (ATCC10721) and Pseudomonas aeruginosa (ATCC10205) were employed. The bacteria were cultivated on Mueller Hinton Agar (Merck, Germany) at 37°C for 24 hrs.
Determination of antibacterial activity
The disk diffusion method was performed using the standard procedure (CLSI) [19]. The three extracts in 5 dilutions (6.25, 12.5, 25, 50, 100 mg /ml) were tested by the disk diffusion method. Bacterial suspensions with a turbidity equivalent to 0.5 McFarland (1.5×108) CFU/ml in BHI broth (Brain Heart infusion broth) (Merck, Germany) were prepared. Then, the suspension was cultured on Muller Hinton Agar (Merck, Germany) [18], [19], [20], [21]. The prepared disks of the different concentrations were placed on the medium. Then, disks containing DMSO were used as the negative control and disks with various antibiotics Penicillin (10 mg), Gentamicin (10 mg) or Vancomycin (30 mg) (Mast England) were used as the positive controls. The plates were incubated at 37°C for 24 hrs. Finally, the zones of inhibition were determined [18], [19], [20], [21].
Extracts that showed potent antibacterial activity were further tested to determine the Minimum Inhibitory Concentration (MIC) by the broth microdilution method. To determine the MIC of the extracts, a solution of 5 mg/ml triphenyltetrazolium (TPTZ) chloride was used. The test was repeated three times to obtain a mean value [22].
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 20, ANOVA and Duncan’s test were used to analyze the data.
Results
Disk diffusion method
The alcoholic extract showed more effective antibacterial action compared to the other extracts (Table 1 [Tab. 1], Table 2 [Tab. 2]). The activity between samples of spring and summer did not show significant differences.
Table 1: Zone of inhibition of alcoholic, hydroalcoholic and aqueous extracts of D. mucronata in springtime (each n=1)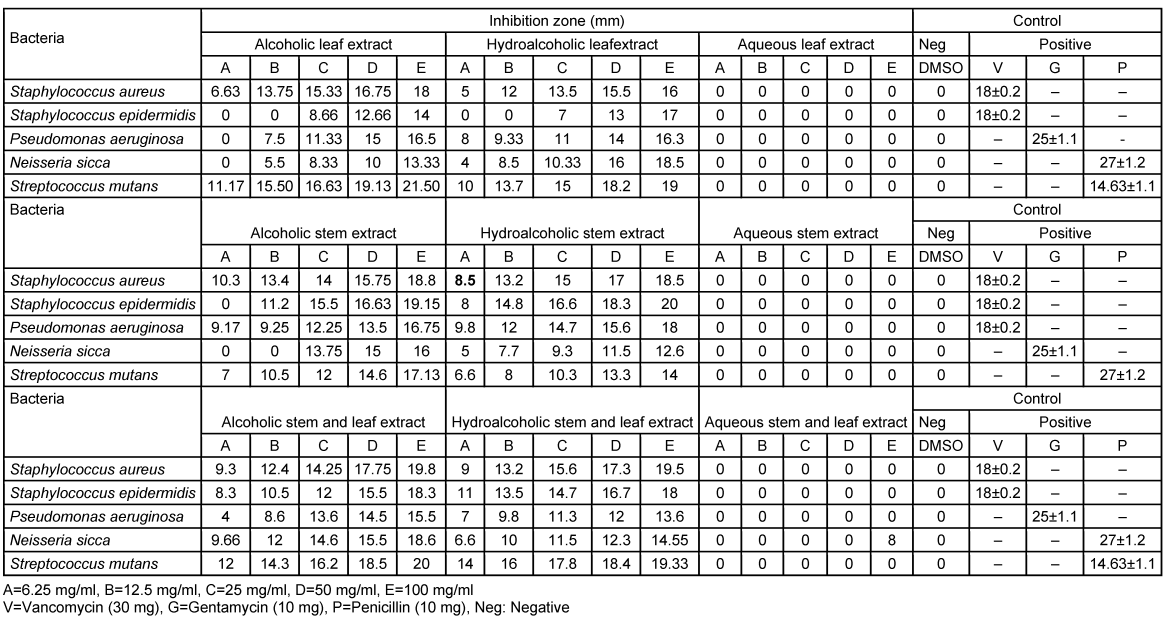
Table 2: Zone of inhibition of the alcoholic, hydroalcoholic, aqueous extracts of D. mucronata harvested in summer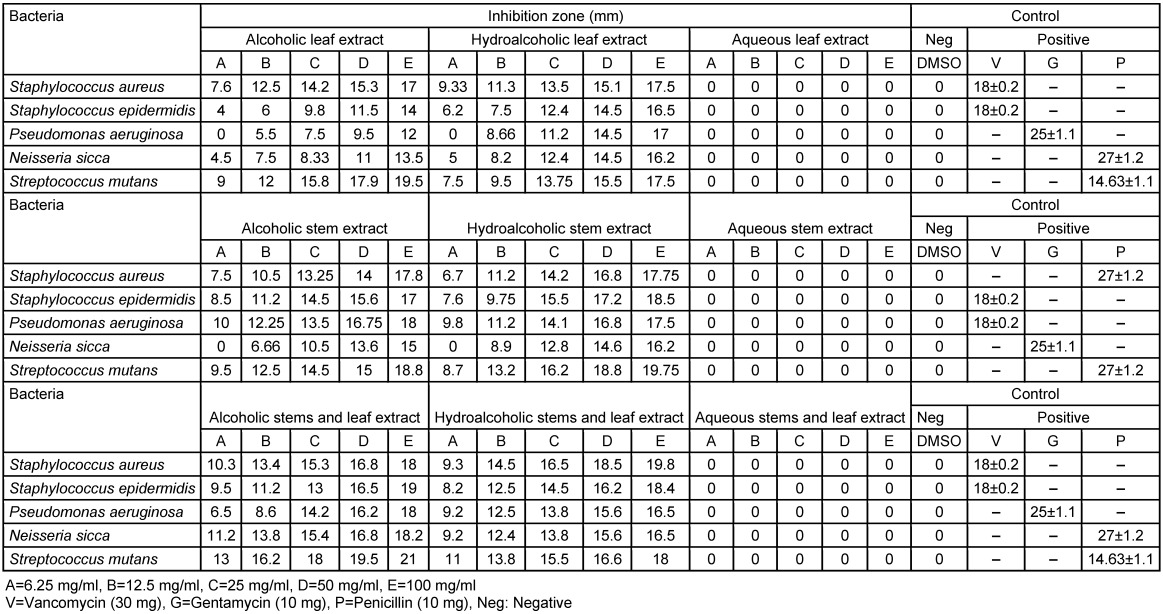
Minimum inhibitory concentration
The alcoholic extract of leaves and stems was the most effective on Streptococcus mutans at a concentration of 0.19 ppm, and the least effective on Staphylococcus epidermidis at concentrations of 12.5 ppm. The hydroalcoholic extract of leaves and stems showed the highest activity on S. aureus and S. mutans at concentrations of 3.12 and 6.25 ppm, respectively, and the lowest activity on S. epidermidis and N. sicca at a concentration of 12.5 ppm (Table 3 [Tab. 3]).The aqueous extract of leaves and stems did not show antimicrobial activity.
Table 3: Results (mean) of minimum inhibitory concentration (MIC) in different dilutions of Daphne mucronata extracts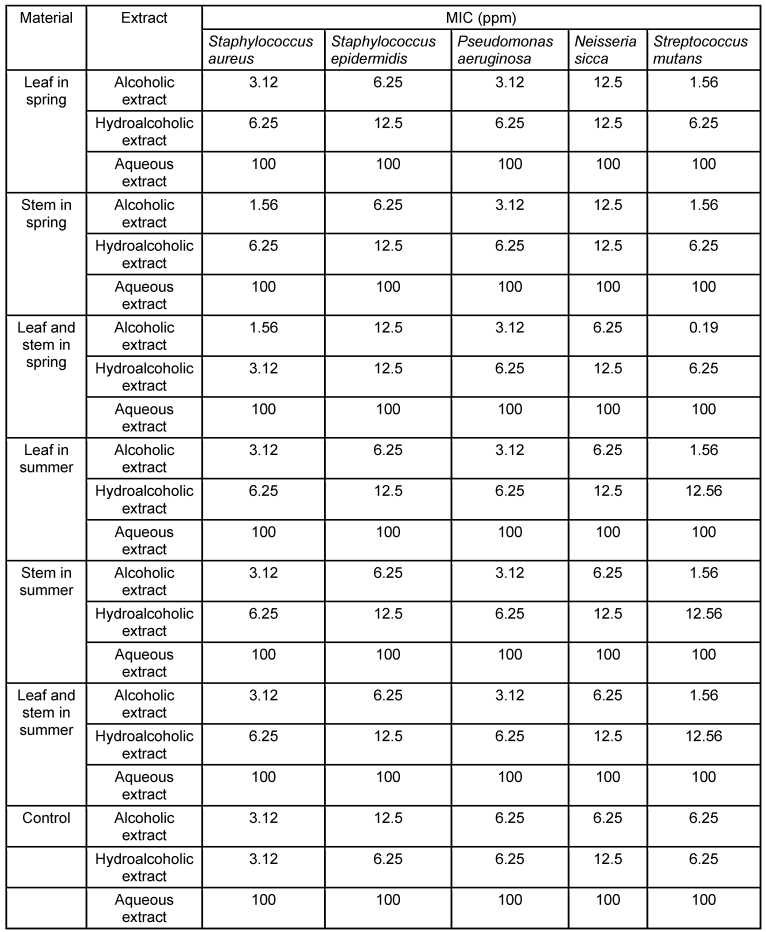
Discussion
Because of the increasing antibiotic resistance rates, manufacturing a new antimicrobial compound is a priority for researchers [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. Since medicinal plants have fewer side effects and are less expensive, they can be used as a source of antibiotics. The diverse geographical and climatic conditions in Iran have brought forth a rich and varied flora. Many of these plants have medicinal properties, such as antibacterial activity [24]. The use of plants of the family Thymelaeaceae has a long history of treating diseases. The genus Daphne is the most medicinally imporant taxon and the most widely used [6], [7], [8], [9], [10]. The highest antimicrobial potentials were observed for the alcoholic extract of leaves. S. mutans was most sensitive species, and the most resistant bacteria were S. epidermidis and P. aeruginosa. In agreement with a study by Tayoub et al. [3] our results showed biological activity of the leaves and stems. The results of the study by Javidnia et al. showed bioactive compounds in roots, stems and leaves of Daphne species [12], which were similar to the results of the present study. Other studies on the genus Daphne showed that the ethanol extract of the stems and leaves contains compounds with antibacterial properties. The study by Tayoub et al. demonstrated the antibacterial effect of an ethanol extract of the leaves and stems of Daphne oliefolia Lam against Escherichia coli, Bacillus subtilis, and P. aeruginosa, while Javadnia et al. found antibacterial and antifungal activity of an ethanolic extract of the leaves and stems of Daphne mucronata against four species of Gram-positive and Gram-negative bacteria [3]. The results obtained by Javidnia showed that ethanolic extracts were active against Escherichia coli and S. aureus, but ethanolic root extracts were the most effective against S. aureus and Bacillus subtilis [12]. In that study, the leaf and stem extract of Daphne mucronata had no effect on P. aeruginosa even at high concentrations; our findings were similar. Abidi et al. [23] found anti pseudomonal activity of Daphne mucronata 5% aqueous extracts using the disk diffusion assay. Daphne mucronata produced a 12 mm zone of inhibition, a biofilm reduction of 40.1%, and biofilm removal of 46% [17]. Cottiglia et al. showed the antibacterial effect of Daphne gnidium L. against different types of bacteria [11]. The stems of Daphne gnidium L. contain 4- coumarin and 7-flavonoid, which could explain the antibacterial activity [11]. A different study also showed that the leaves of Daphne ginidium contain flavonoids and phenolic compounds [17].
Conclusion
Antimicrobial activity studies have shown that Daphne mucronata is very suitable for pharmacognostic and phytochemical studies. Future biochemical studies should test the effective extracted compounds from Daphne mucronata in various diseases.
Notes
Competing interests
The authors declare that they have no competing interests.
References
[1] Houshmand B, Mortazavi H, Alikhani Y, Abdolsamadi H, Ahmadimotemayel F, Zaremahmoudabadi R. In vitro evaluation of antibacterial effect of myrtus extract with different concentrations on some oral bacteria. J Mashad Dent Sch. 2011;35(2):123-30.[2] Kasper DL, Fauci AS. Harrison's Infectious Diseases. 2nd ed. New York: McGraw-Hill; 2013.
[3] Tayoub G, Abu Alnaser A, Shamma M. Microbial inhibitory of the Daphne oleifolia lam. ethanolic extract. Int J Med Arom Plants. 2012;2(1):161-6.
[4] Buhner SH. Herbal Antibiotics: Natural Alternatives for Treating Drug-resistant Bacteria. 2nd ed. Storey Publishing; 2012.
[5] Buhner SH. Herbal Antibiotics: Natural Alternatives for treating Drug-Resistant Bacteria. Vermont: Store Books; 1999.
[6] Hedayati S, Azizi F. The effect of Daphne Mucronata exteract on TNF-α and its receptor on cultured human monocytes. Cell J (Yakhteh). 2005;7(27):152-7.
[7] Kupchan SM, Baxter RL. Mezerein: antileukemic principle isolated from Daphne mezereum L. Science. 1975 Feb 21;187(4177):652-3. DOI: 10.1126/science.1114315
[8] Liou Y, Hall I, Lee K. Antitumor agents LVI: The protein synthesis inhibition by genkwadaphnin and yuanhuacine of P‐388 lymphocytic leukemia cells. J Pharm Sci. 1982;71(12):1340-4. DOI: 10.1002/jps.2600711208
[9] Nasipuri R, Ramstad E. Isolation of Daphnetin-8-β-glucoside from Daphne Papyracea. J Pharm Sci. 1973;62(8):1359-60. DOI: 10.1002/jps.2600620832
[10] Mehdi H, Razieh Y, Marjan Zarif Y, Laleh Hoghooghi R, Fereidoun A. A new diterpene extracted from Daphne mucronata, effects on human K562 and CCRF-CEM cell lines. J Cancer Ther. 2011;2: 71-75. DOI: 10.4236/jct.2011.21008
[11] Cottiglia F, Loy G, Garau D, Floris C, Caus M, Pompei R, Bonsignore L. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomed. 2001;8(4):302-5. DOI: 10.1078/0944-7113-00036
[12] Javidnia K, Miri R, Jahromi RBNNK. A preliminary study on the biological activity of Daphne mucronata Royle. DARU J Pharm Sci. 2003;11(1):28-1.
[13] Satô M, Hasegawa M. Biosynthesis of dihydroxycoumarins in Daphne odora and Cichorium intybus. Phytochem. 1972;11(2):657-62. DOI: 10.1016/0031-9422(72)80029-3
[14] Zhao YX, Huang SZ, Ma QY, Mei WL, Dai HF. Two new daucane sesquiterpenoids from Daphne aurantiaca. Molecules. 2012;17(9):10046-51. DOI: 10.3390/molecules170910046
[15] Rasool MA, Imran M, Nawaz H, Malik A, Kazmi SU. Phytochemical studies on Daphne mucronata. J Chem Soc Pakistan. 2009;31(5):845-50.
[16] Nikitkova AE, Haase EM, Scannapieco FA. Taking the starch out of oral biofilm formation: molecular basis and functional significance of salivary α-amylase binding to oral streptococci. Appl Environm Microbiol. 2013;79(2):416-23. DOI: 10.1128/AEM.02581-12
[17] Abidi S, Ahmed K, Sherwani S, Kazmi S. Reduction and removal of Pseudomonas aeruginosa biofilm by natural agents. Int J Chem Pharm Sci. 2014;5:28-34.
[18] Mahmoudi H, Arabestani MR, Molavi M, Karamolah KS, Fahim NZ. The study effects antimicrobial of Foeniculum vulgare mill and Achilles mille folium plant on bacterial pathogens causing urinary tract infections and nosocomial infection. Int J Pharmacogn Phytochem Res. 2016;8(9):1549-54.
[19] Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptebility Testing: Nineteenth Informational Supplement M100-S19. Wayne, PA: CLSI; 2009.
[20] Mahmoudi H, Arabestani MR, Mousavi SF, Alikhani MY. Molecular analysis of the coagulase gene in clinical and nasal carrier isolates of methicillin-resistant Staphylococcus aureus by restriction fragment length polymorphism. J Glob Antimicrob Resist. 2017;8:41-5. DOI: 10.1016/j.jgar.2016.10.007
[21] Mahmoudi H, Arabestani MR, Mousavi SF, Ghafel S, Alikhani MY. Study of polymorphism spa gene (encoding protein A) of Staphylococcus aureus in clinical isolates and nasal carriers. Tehran Univ Med J TUMS Public. 2015;73(1):24-30.
[22] Jouda MM, Elbashiti T, Masad A, Albayoumi M. The antibacterial effect of some medicinal plant extracts and their synergistic effect with antibiotics. World J Pharm Sci. 2015;5(2):23-33.
[23] Abiri R, Majnooni M, Malek Khattabi P, Adibi H. Study of antimicrobial effects of Trigonella Foenum hydro-alcoholic extract on different bacterial strains. Med Lab J. 2009;3(1):61.
[24] Alizadeh Behbahani B, Tabatabaei Yazdi F, Shahidi F, Mohebbi M. Antimicrobial effect of the aqueous and ethanolic Avicennia marina extracts on Staphylococcus epidermidis, Streptococcus pyogenes and Pseudomonas aeruginosa "in vitro". Iran South Med J. 2014;17(5):879-88.




