[Die ungewöhnliche Infektion: Femoralabszess durch Nocardia bei einem Patienten mit metastasierendem peripheren Bronchialcarcinom und hygienische Konsequenzen]
Georg Daeschlein 1Atteyet-Alla Fetouh Yassin 2
Andreas Franke 3
Axel Kramer 4
Klaus-Peter Schaal 2
1 Department of Dermatology, University Medicine Greifswald, Greifswald, Germany
2 Institute for Medical Microbiology and Immunology of the University of Bonn, Bonn, Germany
3 Specialist Hospital for Pulmonary Medicine and Chest Surgery (FLT) Berlin-Buch, Berlin, Germany
4 Institute for Hygiene and Environmental Medicine, University Medicine Greifswald, Greifswald, Germany
Zusammenfassung
Bei einem 49-jährigen Patienten mit peripherem Bronchialcarcinom mit pulmonaler und zerebraler Metastasierung trat nach Chemotherapie und Bestrahlung ein Femoralabszess auf. Aus der tiefen Oberschenkelwunde links ließen sich insgesamt bei vier Abstrichen Bakterien der Gattung Nocardia über phenotypische und chemotaxonomische Differenzierungsmerkmale nachweisen. Über 16S rRNA Gensequenzierung ließen sich die Isolate als Nocardia abscessus feindifferenzieren. Diese Species wurde kürzlich als Verursacher von Weichteilinfektionen beschrieben. Obwohl selten vorkommend, erfordern Weichteilinfektionen durch Nocardien als Komplikation einer systemischen Nocardiose als typische über Luft übertragene Infektionen immunsupprimierter Patienten besondere Aufmerksamkeit. Die Infektionsprävention erfolgt durch Schutz der Patienten vor Kontakt mit Schmutz und Aerosolen (Baustaub) sowie durch Antibiotikaprophylaxe (Trimethoprim/Sulfamethoxazol).
Schlüsselwörter
Nocardia abscessus, Chemotaxonomie, 16S rRNA Gensequenzierung, Nocardiose, Femoralabscess
Introduction
Due to advances in molecular characterization, the taxonomy of the genus Nocardia has been changing rapidly. This is especially true for the taxon Nocardia asteroides which has been known for many years to be heterogeneous although it comprises the most important human pathogens [16], [22], [23]. Since it is difficult to delineate new species within the classical species Nocardia asteroides solely on the basis of phenotypic characteristics its heterogeneity has long been taken into account by using the collective designation Nocardia asteroides complex [24], [22].
Using a comprehensive phenotypic characterization combined with numerical phenetic analyses Nocardia farcinica was the first species that could reliably be differentiated from the rest of the N. asteroides complex (N. asteroides type B according to Schaal et al. [20]). By the same set of phenotypic data it was also possible to delineate Nocardia cyriacigeorgici [32] as N. asteroides type A1 [20].
Further newly recognized species are N. abscessus (formerly N. asteroides type A2) [20], [31] and N. nova, N. paucivorans, N. africana and N. veterana (all formerly included in the N. asteroides type A3 group) [20], [30]. Among these species N. farcinica is of special importance because of its broad natural resistance to numerous antibiotics, including sulpha drugs and cotrimoxazole. Of further special interest is the pronounced tendency of N. farcinica to disseminate to the brain, kidneys, joints, bone and eyes [21]. Other Nocardia species known to cause human disease are N. brasiliensis, N. otitiscaviarum and N. transvalensis. These species are mainly found in mycetoma lesions and have incidentally been isolated from immunocompromised patients with extensive disease. The distribution and medical significance of Nocardia abscessus [31] as well as the syndromes it can cause, cannot as yet be conclusively evaluated. The following case report shall hence draw attention to N. abscessus in order to be able to close the given scientific gap and to be aware of nocardia infections at immunocompromised patients to start effective prevention measures.
Case Report
Status: 49 year-old patient suspected to have bronchial carcinoma; good general and dietary condition; adapted examination unremarkable both neurologically and medically; no signs of immunosuppression from laboratory chemistry.
History: Longer presence of latent hyperthyroidism in multifocal thyroid autonomy and COPD known.
Course: First hospitalisation with diagnosis of “peripheral bronchial carcinoma of the left superior pulmonary lobe”; histologically macrocellular, poorly differentiated squamous cell carcinoma; classified stage T2 NX.M1.
Computer tomography established right parasagittal cerebral metastasis. Follow-up examinations revealed widespread pulmonary metastases.
Therapy: Cerebral radiation after one month, cytostatic chemotherapy with cisplatin and etoposid 3 resp. 4 month after diagnosis.
Further course: Exacerbation of COPD with partial respiratory insufficiency resulted in emergency admission after half a year. Pneumonic infiltrates were abundant in the region of the left inferior pulmonary lobe upon X-ray. In consequence, high-dose antiobstructive steroid therapy was administered, as well as the antibiotic combination tazobactam/piperacillin. Sputum and blood cultures revealed no microbial growth.
Four weeks post-admission spontaneous an abscess in the left femoral region developed. Sonographically a liquid structure of 4 cm in diameter could be depicted. Upon incision resulted extensive pus emptying. Subsequently repeated antiseptic lavages were performed. This therapy was only followed by a moderate wound healing with formation of granulation tissue and wound closure appeared 8 month after the start of therapy and after multiple surgical local debriding interventions.
Microbiology: Identification was performed at the Actinomycetes Consultant Laboratory at the Institute for Medicinal Microbiology and Immunology of the University of Bonn. Microbiological and molecular biological evidences was given for N. abscessus. The results from routine susceptibility testing revealed susceptible to penicillin, ampicillin, amoxicillin/clavulanic acid, cefotiam, cefuroxime; resistant to trimethoprim/sulfamethoxazole.
In sum, the strain (designated IMMIB D-1873/99) was isolated four times from a deep wound of the left thigh of the patient on columbia blood agar in the course of disease. On this medium the organism formed well developed colonies which consisted of creamy to yellow-coloured substrate mycelia firmly embedded in the agar, and which bore white aerial hyphae. No diffusible pigments were observed. Microscopic examination revealed the presence of filamentous to rod-shaped elements which stained Gram-positive and partially acid fast after staining by the Ziehl-Neelsen method.
Chemotaxonomical analysis of the cell wall components of the isolate revealed all the properties which have hitherto been characteristic of the genus Nocardia. As with all nocardiae it has the cell wall type IV according to Lechevalier and Lechevalier [13] (meso-diaminopimelic acid, arabinose and galactose) as well as nocardomycolic acids. The other chemotaxonomic markers are also typical of nocardiae.
The almost complete 16S rRNA gene sequence (1486 nucleotides) was determined in this study. Sequence comparison using the ARB Database [14] revealed that the 16S rRNA gene sequence of isolate IMMIB D-1873/99 displayed 100% similarity to the reference sequences of N. abscessus (GenBank accession numbers, AF218292, AF218293, and AF218294). Furthermore, phylogenetic analysis using maximum-parsimony, neighbour-joining and maximum likelihood algorithms showed that isolate IMMIB D-1873/99 is closely associated with N. abscessus (Figure 1 [Fig. 1]). These data definitely confirm the identification of isolate IMMIB D-1873/99 as N. abscessus.
Figure 1: Maximium-likelihood tree showing the position of strain IMMIB D-1873/99 within the radiation of species of the genus Nocardia. The tree was based on a comparison of sequences that were at least 90% complete (with regard to E. coli sequence). The numbers at the nodes indicate the level of bootstrap support (%) based on neighbour-joining analyses of 1,000 resampled datasets. The scale bar indicates 10.0% sequence divergence.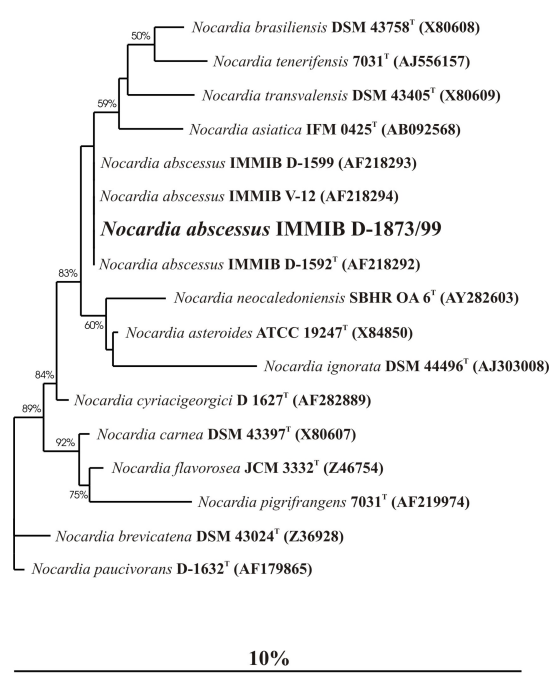
The isolate is physiologically characterized by its ability to utilize citrate, maltose and sucrose as carbon sources and by its inability to hydrolyze esculin (Table 1 [Tab. 1]). Variations in utilizing rhamnose initially led to the consideration that there could exist two different subspecies, as rhamnose utilization by nocardiae in itself is a very stable characteristic. The 16S rDNA sequence analyses, as well as the DNA-DNA pairing results of four different members of the species do not support the delineation of two subspecies, however.
Table 1: Differentiation of pathogenetic Nocardia species based on metabolic-physiological performance [24]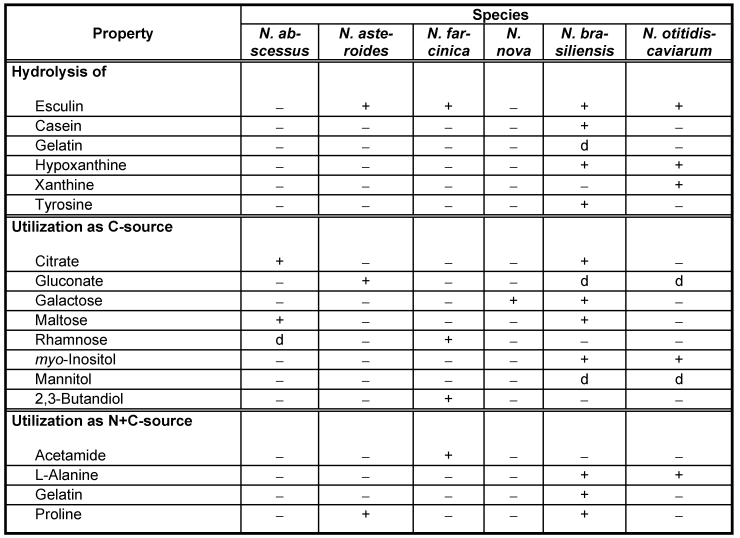
In terms of resistance to antibacterial drugs, N. abscessus behaves like other members of the N. asteroides complex: it is much more susceptible than N. farcinica or N. brasiliensis, for instance [28]. The thigh isolate showed the following susceptibility pattern using microscopic reading of the standard tests applied in this study: susceptible to amoxicillin/clavulanic acid, imipenem, amikacin, gentamicin and tetracyclines; intermediary for tobramycin; resistant to trimethoprim/sulfamethoxazole, vancomycin, erythromycin, ampicillin, cefotaxime and ciprofloxacin. The results look less favourable than those obtained in the routine laboratory but detailed in vitro and in vivo studies [21] have shown that special adaptations of the susceptibility test procedures are required to make in vitro results clinically applicable.
Discussion
Already on the occasion of its first description by Yassin et al. [31] N. abscessus was associated with human disease. Strains of this species were isolated from soft tissue abscesses or empyemas, pericarditis [29], endoprosthesis infection and fibular abscesses [31]. Immunosuppression could not be ascertained in all of the patients involved but seems to be one risk factor for infection, so that particularly in the case of reactivation the infection may take a fatal outcome [6]. The natural habitat of the pathogen as well as its geographical distribution is not known as yet. However, at least one strain was definitely not isolated in Europe; hence worldwide distribution can probably be postulated, as with other representatives of the N. asteroides complex. The same applies to soil as a natural habitat [1], [5], [10]. Because isolates of this species may have been misidentified as N. asteroides on the basis of phenotypic testing, it is quite likely that some isolates previously identified as N. asteroides may actually have been representatives of the species N. abscessus. Further studies to determine the incidence of this species would also help to determine the extent of its involvement in infections at other anatomic sites, its antibiotic susceptibility pattern as well as its geographic distribution and the impact of preventive measures. Analysis of a limited set of phenotypic characteristics and susceptibility testing results usually only allow the recognition of a group of organisms that are evolutionarily rather closely related. Like that of other pathogenetic Nocardia species, the identification of N. abscessus under routine conditions is not entirely straightforward because there is no commercial identification system which produces even approximately reliable results. It is therefore essential to use tests such as hydrolysis of complex substrates and carbon source utilization methods as in-house procedures possibly supplemented with molecular methods (e.g. 16S rDNA sequencing) in order to obtain a reliable identification result at the species level [1], [11], [24]. The members of the species N. abscessus examined so far fail to decompose esculin, casein, gelatin, hypoxanthine, tyrosine, and xanthine; they utilize citrate, maltose and rhamnose as carbon sources, but fail to utilize acetamide, alanine, gelatin, and proline as simultaneous carbon and nitrogen sources; they are susceptible to amoxicillin/clavulanic acid, imipenem, amikacin, gentamicin and tetracyclines. Strain IMMIB D-1873/99 shows biochemical characteristics which match this description.
However, it cannot be excluded that other as yet undescribed species share these characteristics as well. Therefore, for definitive identification of the various species of the N. asteroides complex, molecular methods such as 16S rRNA gene sequencing should be used. The 16S rDNA sequence of isolate IMMIB D-1873/99 shows 100% similarity to the 16S rDNA sequences of the type strain of N. abscessus indicating that strain IMMIB
D-1873/99 is phylogenetically very closely related to or identical with this species.
Infections by N. abscessus have only been reported in five patients up to now in Japan [9], in one German patient who developed a mycetoma after a road accident [6] and in a disseminated infection in an HIV-infected Argentinian patient [3] but the number of cases reported in the literature is increasing [26]. This might be due to an absolute increase in the number of immunocompromised patients but also to improvement in laboratory techniques to detect nocardiosis. Host resistance to nocardial infection depends primarily on the activity of neutrophils and then the cell-mediated immune response. The most common predisposing factors to opportunistic Nocardia infections are long-term steroid usage e.g. in transplant patients, chronic obstructive pulmonary disease (COPD), neoplastic disease, and human immunodeficiency virus (HIV) infection [17], [7].
Our patient represents a further case of infection with unusual Nocardia and the soft tissue infection certainly results from dissemination after primary lung infection (air born infection). No specific risk factor (RKI [12]) (except immunosuppression by lung cancer) regarding Nocardia exposure like close contact to soil and dust contamination can be elicited.
Chemoprophylaxis (of post transplant patients) for preventing urinary tract infections, pneumocystis and Nocardia infections include cotrimoxazole, it is be used for 6–12 months after transplantation, the period of maximum risk for PCP and Nocardia [8], [2] but in our case, this drug showed in vitro resistance to cotrimoxazole and therefore has been omitted for preventive drug therapy. Otherwise, trimethoprim/sulfamethoxazole shows good in vitro activities against most of the unusual Nocardia isolates. Therefore amikazin, as another option for antibiotic infection prevention (and therapy), an antibiotic with also good therapeutic results in Nocardiosis was tested susceptible at our patient and could have been applied [18] .
The second emphasis of preventive measures, the strict restriction of soil and aerosol contact, especially to builder's dust, would have been the hygienic measure of choice for the patient. In the presence of the long course of disease this measure could only be realized regarding his hospitalisation periods but not at all in the meantime at home.
Conclusions
In the light of unusual but often complicated and life-threatening Nocardia soft tissue infections, accurate identification of Nocardia species using molecular techniques is important to make the diagnosis, to elucidate the epidemiology and to start effective therapy as well and accurate prevention measures. The reliable differentiation of N. abscessus from other Nocardia species requires special diagnostic procedures and some experience in handling aerobic pathogenic actinomycetes. The case report presented here describes N. abscessus as a pathogen in endogenous and exogenous immunosuppression (bronchial carcinoma treated with chemotherapy).
Notes
Conflicts of interest
The authors declare that they have no competing interests.
References
[1] Beaman BL, Boiron P, Beaman L, Brownell GH, Schaal K, Gombert ME. Nocardia and nocardiosis. J Med Vet Mycol. 1992;30(Suppl 1):317-31. DOI: 10.1080/02681219280001001[2] de Souza RM, Olsburgh J. Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol. 2008;4(5):252-64. DOI: 10.1038/ncpneph0781
[3] Diego C, Ambrosioni JC, Abel G, Fernando B, Tomás O, Ricardo N, Jorge B. Disseminated nocardiosis caused by Nocardia abscessus in an HIV-infected patient: first reported case. AIDS. 2005;19(12):1330-1. DOI: 10.1097/01.aids.0000180108.96135.25
[4] Gombert ME, duBouchet L, Aulicino TM, Berkowitz LB. Antimicrobial synergism in the therapy of experimental cerebral nocardiosis. J Antimicrob Chemother. 1989;24(1):39-43. DOI: 10.1093/jac/24.1.39
[5] Gordon RE, Barnett DA, Handerhan JE, et al. Nocardia coeliaca, Nocardia autotrophica, and the nocardin strain. Int J Syst Bacteriol. 1974;24: 54-63. DOI: 10.1099/00207713-24-1-54
[6] Horré R, Schumacher G, Marklein G, Stratmann H, Wardelmann E, Gilges S, De Hoog GS, Schaal KP. Mycetoma due to Pseudallescheria boydii and co-isolation of Nocardia abscessus in a patient injured in road accident. Med Mycol. 2002;40(5):525-7.
[7] Hui CH, Au VW, Rowland K, Slavotinek JP, Gordon DL. Pulmonary nocardiosis re-visited: experience of 35 patients at diagnosis. Respir Med. 2003;97(6):709-17. DOI: 10.1053/rmed.2003.1505
[8] Jha V. Post-transplant infections: An ounce of prevention. Indian J Nephrol. 2010;20(4):171-8. DOI: 10.4103/0971-4065.73431
[9] Kageyama A, Yazawa K, Ishikawa J, Hotta K, Nishimura K, Mikami Y. Nocardial infections in Japan from 1992 to 2001, including the first report of infection by Nocardia transvalensis. Eur J Epidemiol. 2004;19(4):383-9. DOI: 10.1023/B:EJEP.0000024706.02325.c0
[10] Kageyama A, Yazawa K, Kudo T, Taniguchi H, Nishimura K, Mikami Y. First isolates of Nocardia abscessus from humans and soil in Japan. Nihon Ishinkin Gakkai Zasshi. 2004;45(1):17-21. DOI: 10.3314/jjmm.45.17
[11] Kiska DL, Hicks K, Pettit DJ. Identification of medically relevant Nocardia species with an abbreviated battery of tests. J Clin Microbiol. 2002;40(4):1346-51. DOI: 10.1128/JCM.40.4.1346-1351.2002
[12] Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut (RKI). Anforderungen an die Hygiene bei der medizinischen Versorgung von immunsupprimierten Patienten. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut (RKI). BgBl. 2010;53:357-88. DOI: 10.1007/s00103-010-1028-9.
[13] Lechevalier MP, Lechevalier HA. Chemical composition as a criterion in the classification of aerobic actinomycetes. Int J Syst Bacteriol. 1970; 20:435-43. DOI: 10.1099/00207713-20-4-435
[14] Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Förster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüssmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32(4):1363-71. DOI: 10.1093/nar/gkh293
[15] McNeil MM, Brown JM, Hutwagner LC. Evaluation of therapy for Nocardia asteroides complex infections. Infect Dis Clin Pract. 1995;4:287-92. DOI: 10.1097/00019048-199507000-00011
[16] McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7(3):357-417.
[17] Menéndez R, Cordero PJ, Santos M, Gobernado M, Marco V. Pulmonary infection with Nocardia species: a report of 10 cases and review. Eur Respir J. 1997;10(7):1542-6. DOI: 10.1183/09031936.97.10071542
[18] Muñoz J, Mirelis B, Aragón LM, Gutiérrez N, Sánchez F, Español M, Esparcia O, Gurguí M, Domingo P, Coll P. Clinical and microbiological features of nocardiosis 1997-2003. J Med Microbiol. 2007;56(Pt 4):545-50.
[19] Rainey FA, Burghardt J, Kroppenstedt RM, et al. Phylogenetic analysis of the genera Rhodococcus and Nocardia and evidence for the evolutionary origin of the genus Nocardia from within the radiation of Rhodococcus species. Microbiol. 1995;141:523-8. DOI: 10.1099/13500872-141-2-523
[20] Schaal KP, Reuterberg, H. Numerical taxonomy of Nocardia asteroides. Zbl Bakteriol Parasitol Infekt Hyg I Abt. 1978;6:53-62.
[21] Schaal KP, Schutt-Gerowitt H, Goldman A. In vivo and in vitro studies on the efficacy of various antimicrobial agents in the treatment of human nocardiosis. In: Szabo G, Miro S, Goodfellow M, eds. Biological, biochemical, and biomedical aspects of Actinomycetes. Part B. Budapest: Academia Kiado; 1986. p. 619-33.
[22] Schaal KP. Actinomycoses, actinobacillosis and related diseases. In: Collier L, Balows A, Sussman M, eds. Topley & Wilson's Microbiology and Microbial Infections. London: Arnold; 1998. p. 777-98.
[23] Schaal KP. Nocardiosen. In: Domschke W, Hohenberger W, Meinertz T, et al., eds. Therapie-Handbuch. München: Urban Schwarzenberg; 2000. p. S3-64-6.
[24] Schaal KP. Pathogene aerobe Aktinomyzeten. In: Burkhardt F, ed. Mikrobiologische Diagnostik. Stuttgart: Thieme; 1992. p. 258-68.
[25] Steingrube VA, Brown BA, Gibson JL, Wilson RW, Brown J, Blacklock Z, Jost K, Locke S, Ulrich RF, Wallace RJ Jr. DNA amplification and restriction endonuclease analysis for differentiation of 12 species and taxa of Nocardia, including recognition of four new taxa within the Nocardia asteroides complex. J Clin Microbiol. 1995;33(12):3096-101.
[26] Tan CK, Lai CC, Lin SH, Liao CH, Chou CH, Hsu HL, Huang YT, Hsueh PR. Clinical and microbiological characteristics of Nocardiosis including those caused by emerging Nocardia species in Taiwan, 1998-2008. Clin Microbiol Infect. 2010;16(7):966-72.
[27] Walensky RP, Moore RD. A case series of 59 patients with nocardiosis. Infect Dis Clin Pract. 2001;10:249-54. DOI: 10.1097/00019048-200106000-00003
[28] Wallace RJ Jr, Steele LC. Susceptibility testing of Nocardia species for the clinical laboratory. Diagn Microbiol Infect Dis. 1988;9(3):155-66. DOI: 10.1016/0732-8893(88)90025-9
[29] Wellinghausen N, Pietzcker T, Kern WV, Essig A, Marre R. Expanded spectrum of Nocardia species causing clinical nocardiosis detected by molecular methods. Int J Med Microbiol. 2002;292(3-4):277-82. DOI: 10.1078/1438-4221-00208
[30] Yassin AF, Rainey FA, Burghardt J, Brzezinka H, Mauch M, Schaal KP. Nocardia paucivorans sp. nov. Int J Syst Evol Microbiol. 2000;50(Pt 2):803-9. DOI: 10.1099/00207713-50-2-803
[31] Yassin AF, Rainey FA, Mendrock U, Brzezinka H, Schaal KP. Nocardia abscessus sp. nov. Int J Syst Evol Microbiol. 2000;50(Pt 4):1487-93. DOI: 10.1099/00207713-50-4-1487
[32] Yassin AF, Rainey FA, Steiner U. Nocardia cyriacigeorgici sp. nov. Int J Syst Evol Microbiol. 2001;51(Pt 4):1419-23.




