[Dekontamination der Innenraumluft und angrenzender Wandflächen durch Wasserstoffperoxid-Verneblung]
Torsten Koburger 1Harald Below 2
Tina Dornquast 2
Axel Kramer 2
1 Hygiene Nord GmbH, Greifswald, Germany
2 Institute of Hygiene and Environmental Medicine, University Medicine, Greifswald, Germany
Zusammenfassung
Zielsetzung: Zur Verneblung von Wasserstoffperoxid (H2O2) wurde 2010 das ASP GLOSAIRTM 400 in Deutschland eingeführt. Da hierzu bisher keine Ergebnisse einer Praxiserprobung vorliegen, sollte das neue Verfahren auf Wirksamkeit zur Raumluftdekontamination in Räumen mit Schimmelpilzbefall nach Wasserschaden unter Praxisbedingungen und parallel unter experimentell kontrollierten Bedingungen auf mit Aspergillus brasiliensis kontaminierten Testflächen geprüft werden.
Methode: In zwei Räumen mit massivem Schimmelpilzbefall wurden nach Einsatz des Geräts die Luftkoloniezahl bestimmt (MAS-100® Zinsser Analytik) und parallel in Proben von Wandputz der Schimmelpilzbefall bestimmt.
Im Rahmen der kontrollierten Studie wurden im Testraum vertikal und horizontal mit Aspergillus brasiliensis kontaminierte Testflächen positioniert und auf diesen gemäß EN 13697 die Effektivität der Verneblung (5–6 % H2O2 für 2 h) geprüft.
Ergebnisse: Bei massivem Pilzbefall nach einem Wasserschaden (worst case) war unmittelbar nach Beendigung der Verneblung eine etwa 9-fache Absenkung des Schimmelpilzgehalts und eine etwa 13-fache Absenkung der Anzahl Koloniebildender Einheiten (Summe von Bakterien + Pilzen) in der Raumluft nachweisbar. Auch in Proben von Wand- und Fugenputz wurden die Schimmelpilze reduziert, wenn auch in deutlich geringerem Ausmaß.
Bei Vernebelung von 5–6% H2O2 wurde A. brasiliensis um >4 log auf vertikalen und horizontalen Testflächen reduziert.
Diskussion: In Räumen mit massivem Schimmelpilzbefall ist das ASP GLOSAIRTM 400 System geeignet, eine deutliche für etwa eine Woche anhaltende Reduzierung der fungiellen und bakteriellen Raumluftbelastung zu erreichen. Auf experimentell mit A. brasiliensis kontaminierten vertikalen und horizontalen Flächen wird eine Reduktion von >4 lg erreicht. Da eine Kontamination von 104–105 Pilzsporen nach Vorreinigung von Flächen unwahrscheinlich ist, kann das GLOSAIRTM 400 als geeignetes ergänzendes Verfahren zur wirksamen Reduktion einer Pilzkontamination angesehen werden.
Schlüsselwörter
Wasserstoffperoxid, Vernebelung, ASP GLOSAIR™ 400, Schimmelpilzsanierung
Introduction
There frequently is mold infestation in rooms with water damage or moisture problems. In such a case, the danger exists that the health of the room occupants will be affected or that there will be damage to the building substance [1], [2], [3].
The most important action in the event of a mold infestation is to dry the building rapidly and to remove the mold infestation in the room air and on the room surface quickly and expertly. For this purpose, there are different methods, which use different active agents. A careful consideration of risks and benefits is of decisive importance for selecting a method. The vaporization of formaldehyde is to be rejected especially for toxicological reasons [4]. The same is evidently true also for quaternary ammonium compounds, even though these materials have been investigated less intensively so far [5]. Nebulizing hydrogen peroxide is a toxicologically safe and, nevertheless, effective alternative [6]. When an aqueous solution of 7.5% hydrogen peroxide (H2O2) and 0.0075% silver is nebulized as a finely dispersed aerosol at a relative humidity of 50% and a temperature of 29°C and allowed to act for six hours, an effectiveness of >4 log was achieved on wetted surfaces under “clean” (contaminated with 0.03% albumin) as well as under “dirty” conditions (contaminated with 0.3% albumin as well as with sheep erythrocytes) vis-a-vis S. aureus, E. hirae, P. aeruginosa, A. brasiliensis, M. terrae, M. avium and spores of B. subtilis [7]. The use of H2O2 without silver is even more advantageous toxicologically.
The ASP GLOSAIRTM 400 was introduced in Germany in 2010 for nebulizing H2O2. Since the results of testing under practical conditions had not been published previously, the new method was to be tested for effectiveness in decontaminating the air in rooms with a mold infestation after water damage under practical conditions and, at the same time, under experimentally controllable conditions on test surfaces, contaminated with Aspergillus brasilliensis and set up vertically and horizontally in the room.
Methods
Description of the nebulizing process
The ASP GLOSAIRTM 400 (Advance Sterilization Products Norderstedt, Germany) sprays H2O2 solution (5–6%) and silver cations (50 ppm) as a mist and can be used for rooms between 10 m3 and 200 m3 in size. The antimicrobial agent is provided in a sealed, replaceable cartridge which is automatically drained into an internal reservoir. The user inputs the room size to be disinfected and the GLOSAIRTM 400 adjusts the solution volume to be sprayed in accordance with that. The GLOSAIRTM 400 process comprises three phases. The first phase is diffusion during which the GLOSAIRTM Solution is converted from a liquid to a mist with an average particle size from 8–12 µm to a concentration of about 100 ppm. The second phase is the contact time during which the mist interacts with the contaminating organisms on the room surfaces. The third and final phase is aeration to reduce any remaining disinfectant concentration to safe levels for human re-entry into the room. The entire process takes about 3 hours depending on room size and may be controlled from outside the room via a wireless remote control.
Testing after mold infestation resulting from water damage
Room Situation
Increased mold values were detected in the air of rooms with moisture damage. In room 1, the masonry in the window region had been soaked for some years and was infested with mold. The user of the room complained of health problems, which could obviously be attributed to the mold infestation. At the time of the measurements, the room was being structurally renovated. Facilities, the interior plaster of the window region and a portion of the right wall had been removed, so that the construction substance was exposed there. At the time of the measurement, the drying of the building had already gone on for some weeks, but had not yet been concluded (residual moisture in the window wall 5–10%). For testing the decontamination effect, the drying of the building was interrupted. The room was not cleaned before it was nebulized, that is, there was a considerable amount of dust. During the measurements, the room was not used and entered only by the measurement personnel.
In 2010, a pipe in room 2 broke and there was water damage. The room was renovated without being dried and had an increased content of mold in the room air. Use of the room was continued after the nebulizing process that is, the room was entered and vented as required.
The disinfectant was sprayed in an amount of 6 mL per m3. In both rooms, the equipment was positioned in the room so that the jet of mist was directed diagonally in the room (Figure 1 [Fig. 1]). At the end of the spraying phase (5–10 min.) a period of action of 2 hours was maintained, during which the room was not entered.
Figure 1: Setting up the nebulizing equipment
Determining the air colony count
The effect of the nebulization was determined by estimating the mold and bacterial counts in the room air before and after the treatment. The MAS 100® (Zinsser Analytik) air colony counter was used for the sampling. The sample volume was 250 L. Columbia blood agar was used as a nutrient for detecting especially bacteria and Sabouraud agar for detecting fungi. Immediately after the sampling, the agar plates were brought into the laboratory and incubated there for two days at 37°C and for a further five days at 22°C.
Sampling the wall plaster
Plaster samples were filled with a spatula into a sterile tube. Material (1 g) was suspended in 9 mL of sterile sodium chloride solution and shaken thoroughly, after which 100 µL were transferred to Sabouraud agar and incubated at 22°C for seven days.
Testing of fungicidal efficiency on contaminated carrier
Testing was performed following the European Standard EN 13697 [8] with Aspergillus brasiliensis (formerly named A. niger) as test-organisms. In deviation from that standard, the germ carriers were distributed in the test room (Figure 2 [Fig. 2]). The test surfaces at the walls were placed vertically with the contaminated side of the carrier facing the room (positions 1 and 5). Test surfaces at the remaining positions were placed horizontally with the contaminated side of the carrier facing up; only at position 8 (ceiling) the contaminated side was facing down (towards the room).
Figure 2: Positions of the test surfaces in the test room (two carriers per position) (1) Wall behind GLOSAIRTM 400, 1.30 m above the floor (2) Right side behind GLOSAIRTM 400, 2.00 m above the floor, on top of fuse box (3) Floor next to GLOSAIRTM 400, 1.00 m away (4) Floor directly in front of GLOSAIRTM 400 (5) Wall to the right of and about 2.00 m away from GLOSAIRTM 400, 1.70 m above the floor (6) On top of a cardboard box (0.40 m) to the left of and about 2.50 m away from GLOSAIRTM 400 (7) Floor around the corner in front of locker, about 4.00 m away from GLOSAIRTM 400 (9) Left side in front of GLOSAIRTM 400, 2.00 m above the floor, on top of locker, about 4.20 m away from GLOSAIRTM 400 (10) Wall opposite of GLOSAIRTM 400, 1.70 m above the floor, about 5.00 m away from GLOSAIRTM 400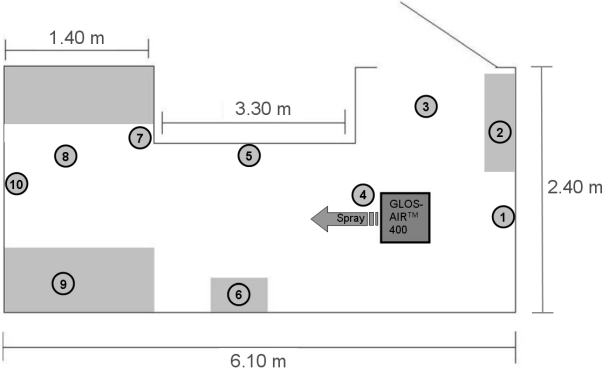
After initial screening with higher concentrations of the test organism/carrier, 104–105 were applied (instead of the EN 13697 standard concentration of 105–106 test organism/carrier) in order to account for the complementary nature of the GLOSAIRTM 400 Area Decontamination System outlined above. The cubic space of the test room was determined to be 37 m³.
For the test, small metal plates (20 mm diameter of EN 10088 stainless steel [9]) are used as test surfaces. Plates had been boiled in a soft soap solution for 60 min, washed with tap water and afterwards rinsed with distilled water. The plates were disinfected with 2-propanol (70% v/v) for 15 min. After drying, the plates were used for the test or stored under aseptic conditions.
For contamination, 0.05 mL of the test organism suspension with addition of 0.03% albumin (clean conditions) are pipetted on the plates and spread on the central area of the test surfaces.
After drying ≤1 hour, the test plates are distributed in the test room and the GLOSAIRTM 400 process is started. During the entire process, the test room is kept shut and the ventilation system is kept shut off. At the end of the required contact time, the metal plates are transferred to tubes containing glass beads in 10 mL of sterile TSB with neutralizer (3% tween 80+0.3% lecithin+0.1% histidine+0.5% sodium thiosulfate), which is known to be efficient for inactivating the hydrogen peroxide. Those tubes are then vortexed for 2 min to release the remaining viable test organisms from the test surfaces. Aliquots of the resulting test-neutralisation-solution and its dilutions were plated using the pour-plate technique. The reduction (ME) is calculated in relation to a control test surface treated with water instead of the test product (Nc).
The test was performed at room temperature (20±1°C). The test organisms are incubated at 30±1°C.
Results
Effectiveness for mold infestation after water damage
Immediately after the nebulization in room 1 was concluded, it was possible to identify a 9-fold decrease in the mold content and an approximately 13-fold decrease in the number of colony-forming units (sum of bacteria + fungi) (Table 1 [Tab. 1], Figure 3 [Fig. 3]).
Table 1: Mold Infestation and colony-forming units (sum of bacteria and fungi) before and after nebulization with H2O2 in room 1 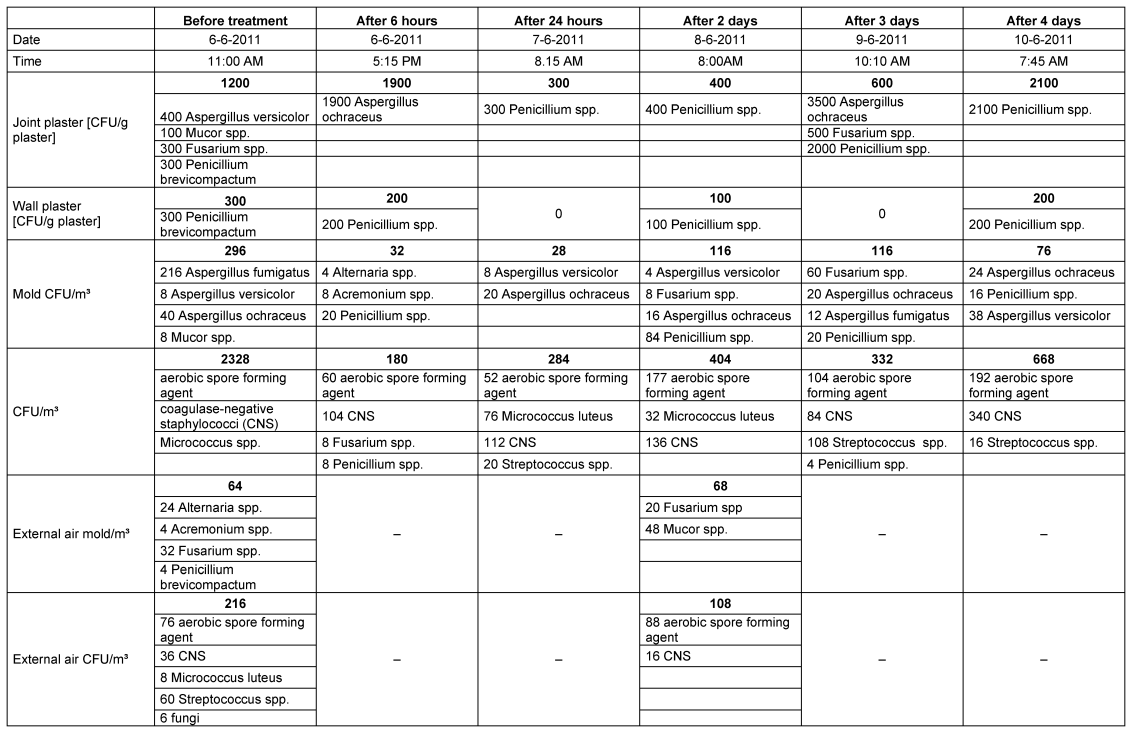
Figure 3: Mold/m3 and bacterial + fungal (CFU)/m3 in the air of room 1 before and after decontamination 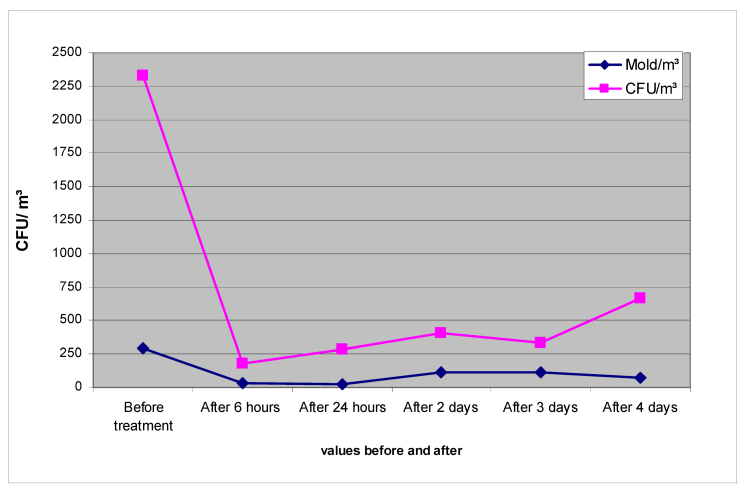
The reduction in mold could also be detected in samples from wall and joint plaster, even though it was much less pronounced (Table 1 [Tab. 1], Figure 4 [Fig. 4]). Evidently, the depth effect of the hydrogen peroxide mist is limited.
Figure 4: Mold (CFU)/g of plaster in room 1 before and after decontamination with ASP GLOSAIRTM 400 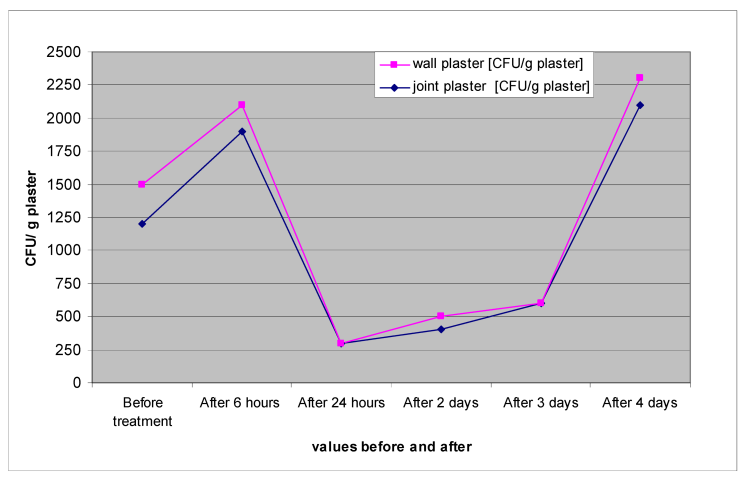
In comparison to room 1, there were two significant differences in room 2 after the nebulization. The reduction in the total value of bacteria and fungi did not start immediately after the nebulization; instead, it was delayed. After that, the mold infestation decreased continuously; the total values decreased only briefly and then remained constant at a relatively high level (Table 2 [Tab. 2], Figure 5 [Fig. 5]).
Table 2: Mold infestation and colony-forming units (sum of bacteria and fungi) before and after nebulization with H2O2 in room 2 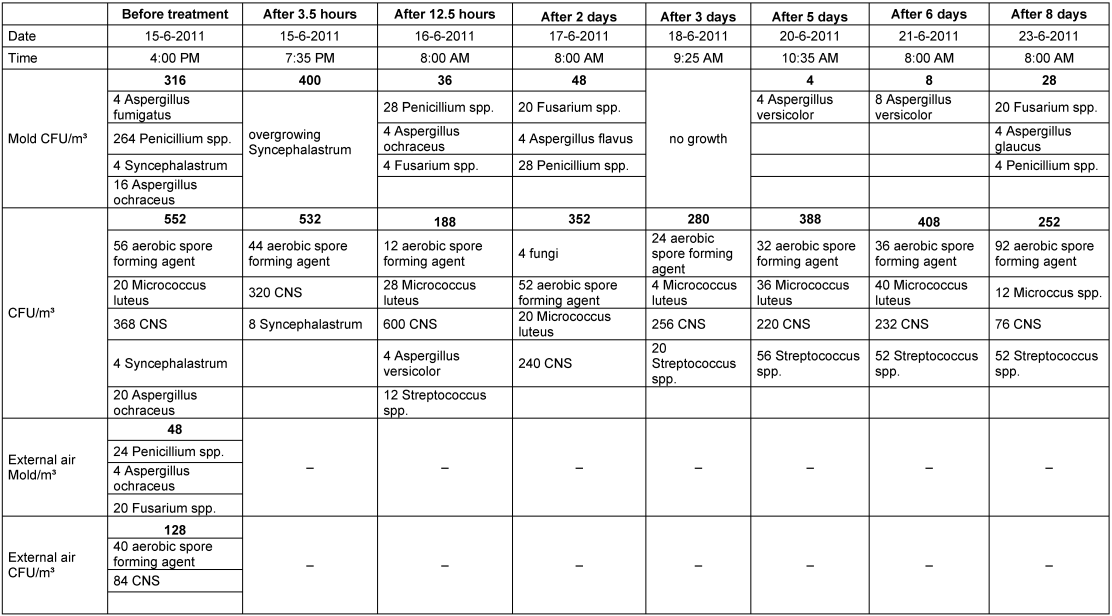
Figure 5: Mold/m3 and bacterial + fungal (CFU)/m3 in the air of room 2 before and after decontamination 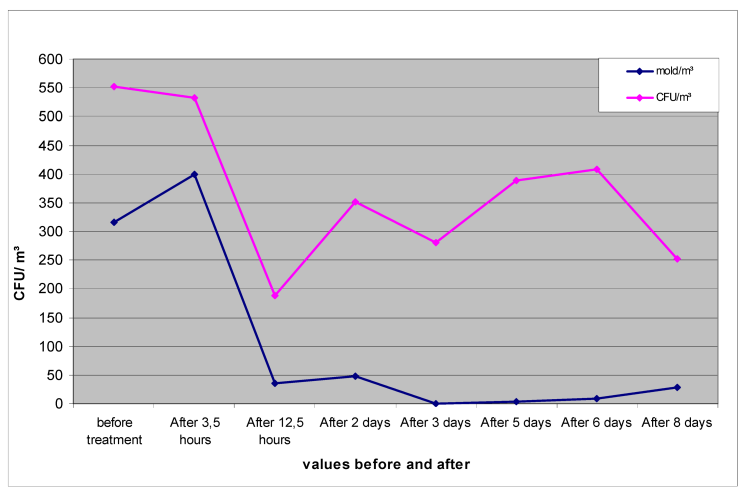
Effectiveness on contaminated surfaces
To begin with, the effectiveness of the nebulization as a function of the amount of contamination of the test organism (A. brasiliensis ATCC 16404) on three test surfaces, distributed in the room, was investigated. The required disinfection effectiveness on surfaces of ≥4 log was achieved only when the bioburden was 4.28 log (Table 3 [Tab. 3]). At a higher contamination, this value was clearly not reached (Table 3 [Tab. 3]).
Table 3: Effectiveness as a function of the bioburden for a period of action of 2 hours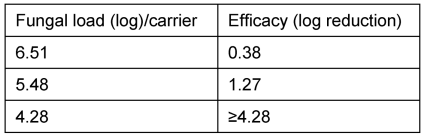
For this reason, the main testing was carried out with a load of the order of 4 log. A reduction of ≥4.46 was achieved here on all test surfaces, that is, with vertical as well as with horizontal positioning (Nos. 1–10) with a time of action of 2 hours.
Discussion
ASP GLOSAIRTM 400
The system is not intended to be a complete replacement for manual cleaning and surface disinfection; rather, it is a complementary process to reduce risk in areas where contamination is likely to occur. It also adds additional hygienic safety to areas that are difficult to disinfect or hard to access by manual disinfection.
Effectiveness for mold infestation
Because of the considerable amount of dust present, room 1 represents a worst case scenario. In half of the room, the wall plaster was removed and the masonry exposed. In the rest of the room, the wallpaper had largely been removed. Construction dust and floor coverings were not removed during the measurement and the room was not cleaned thoroughly (although this is done usually) before the nebulization (Figure 6 [Fig. 6]). In spite of these more difficult conditions, a reduction in mold in the room air could be detected for the period of seven days. The same was the case with the number of CFU (Table 1 [Tab. 1]). These findings are remarkable also because the residual moisture content of the window wall was still appreciable, while the outside temperature during the measurement period ranged from 20° to 30°C and the room was not vented. In other words, the conditions were ideal for renewed mold growth. The mold content of the outside air was 64 molds/m3 and the sum of the colony-forming units of bacteria and fungi was 216 CFU/m3.
Figure 6: Condition in room 1 during the nebulization
It was possible to show in room 2 that nebulization leads to a clear reduction in the mold content in the room air even in rooms, which are being used (Figure 7 [Fig. 7]). The reason why, in comparison to room 1, the onset of the reduction of the air flora was delayed, may well have been the large area of the mold infestation, which affected 4 rooms, which were connected to one another over a common corridor and separated only by doors. Measurements were carried out only in room 1. The other three rooms were decontaminated at daily intervals, that is, the rehabilitation of the affected rooms was completed only on day 3. During this time, room users moved between treated and untreated rooms. There was a measurable effect only after two of the rooms had been treated. With respect to mold, this effect was comparable to that in room 1. The fact that the CFU values remained at a high level may be attributed to the use of the rooms, with the result that microflora were brought in continuously by the users.
Figure 7: Furnishings in room 2 with the GLOSAIRTM 400 set up
Conclusions
In rooms with a massive mold infestation, the ASP GLOSAIRTM 400 is suitable for achieving a week-long reduction in the fungal and bacterial room air load. At the same time, a reduction of A. brasiliensis >4 lg is achieved on the vertical and horizontal surfaces. As a load of 104 to 105 fungal spores is unlikely to occur on pre-cleaned surfaces, the GLOSAIRTM 400 can be considered a suitable complementary process for the substantial reduction of fungal contaminations.
Notes
Acknowledgement
Funding sources for in situ studies: none.
Funding sources for laboratory studies: Johnson & Johnson Medical GmbH, ASP.
Conflict of interest statement: The laboratory study was performed by Hygiene Nord, Mr. Koburger.
References
[1] Moriske HJ, Szewzyk R, Umweltbundesamt Innenraumlufthygienekommission. Leitfaden zur Vorbeugung, Untersuchung, Bewertung und Sanierung von Schimmelpilzwachstum in Innenräumen („Schimmelpilz-Leitfaden“). Berlin: Umweltbundesamt; 2002.[2] Moriske HJ, Szewzyk R, Umweltbundesamt Innenraumlufthygienekommission. Leitfaden zur Ursachensuche und Sanierung bei Schimmelpilzwachstum in Innenräumen („Schimmelpilzsanierungs-Leitfaden“). Dessau: Umweltbundesamt; 2005.
[3] WHO Guidelines for indoor air quality. Dampness and Mould. Copenhagen: World Health Organization; 2009.
[4] Kramer A, Pitten FA, Reichwagen S. Aerosolanwendung mikrobizider Wirkstoffe. In: Kramer A, Assadian O, eds. Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung: Qualitätssicherung der Hygiene in Industrie, Pharmazie und Medizin. Stuttgart: Thieme; 2008. p. 905.
[5] Kramer A. Gesundheitliche Risiken der Flächendesinfektion am Beispiel von quaternären Ammoniumverbindungen (Quats) und Konsequenzen für den Einsatz in Wohnräumen. Bericht 15. Pilztagung, Fürth: AnBUS e.V., 2011, 113-120.
[6] Kramer A, Reichwagen S, Heldt P, Widulle H, Nürnberg W. Oxidanzien. In: Kramer A, Assadian O, eds. Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung: Qualitätssicherung der Hygiene in Industrie, Pharmazie und Medizin. Stuttgart: Thieme; 2008. p. 713-745.
[7] Pitten FA. Wasserstoffperoxid. In: Kramer A, Assadian O, eds. Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung: Qualitätssicherung der Hygiene in Industrie, Pharmazie und Medizin. Stuttgart: Thieme; 2008. p. 905.
[8] EN 13697. Chemical disinfectants and antiseptics – Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas – Test method and requirements without mechanical action (phase 2, step 1). Berlin: Beuth; 2001.
[9] EN 10088-1. Nichtrostende Stähle – Teil 1: Verzeichnis der nichtrostenden Stähle [Stainless steels – Part 1: List of stainless steels]. Berlin: Beuth; 2005.




