[Muss das Infusionssystem bei Kurzzeitapplikation von Antibiotika mit jedem Wechsel der Infusionsflasche gewechselt werden?]
Felix von Au 1Sylvia Ryll 1
Christian Wegner 1
Stephan Gessner 1
Axel Kramer 1
1 Institute of Hygiene and Environmental Medicine, University Medicine, Ernst Moritz Arndt University, Greifswald, Germany
Zusammenfassung
Zielsetzung: Gemäß Herstelleranweisung ist bei jedem Wechsel der Infusionsflasche der Wechsel des gesamten Systems erforderlich. Es sollte untersucht werden, ob das bei aufeinanderfolgenden Kurzzeitinfusionen ausschließlich von Antibiotika zutrifft.
Methode: Beim Wechsel des Infusionssystems nach 72 h wurde die im System verbliebene Restlösung nach Inaktivierung mit Eigelb für 48 h bei 36 °C auf Blutagar zum Nachweis einer Kontamination kultiviert.
Ergebnisse: In keiner von 87 untersuchten Proben konnte eine mikrobielle Kontamination nachgewiesen werden. Irrtümlich wurde eine weitere Probe aus einem Zugang entnommen, über den kein Antibiotikum verabreicht wurde. Diese enthielt eine Koloniebildende Einheit von Coagulase-negativen Staphylokokken.
Die Ergebnisse sprechen dafür, dass bei aufeinanderfolgenden Kurzinfusionen von Antibiotika das Infusionsystem beim Wechsel der Infusionsflasche nicht gewechselt werden muss. Sofern nicht ausschließlich Antibiotika verabreicht werden, muss der Wechsel jedoch durchgeführt werden, weil eine irrtümlich entnommene Probe aus einem anderen Zugang mikrobiell kontaminiert war. Zur weiteren Abklärung der Fragestellung wird eine Nachfolgestudie mit Einschluss einer größeren Patientenzahl als erforderlich angesehen.
Schlussfolgerung: Für den nicht-antibiotischen Zugang muss bei jedem Wechsel der Infusionsflasche auch das Infusionsset gewechselt werden. Die Ergebnisse unserer Pilotstudie sprechen jedoch dafür, dass dieser Wechsel nicht erforderlich ist, sofern nur Antibiotika appliziert werden.
Schlüsselwörter
ZVK, Antibiotikakurzinfusion, Wechsel des Infusionssystems, Wechsel der Infusionsflasche
Introduction
Manufacturers’ instructions recommend changing the i.v. administration set together with the infusion bottle after each administration. The recommendation of KRINKO [1] requires similarly, that feed-lines in the case of short infusions must be discarded after the completion of the infusion. However, in practice it is observed in the case of several directly cascaded short infusions for a given drug compatibility usually the same administration line is used.
Particularly in intensive care, a number of different solutions are administered over the limb of the central venous catheter (CVC) daily. Therefore, following the manufacturers’ recommendations is associated with costs and increased workload for healthcare workers.
To the best of our knowledge no studies have investigated if immediate renewing of the complete i.v. administration set together with an emptied infusion bottle is required. Even the very comprehensive CDC's 2011 Guidelines for the prevention of intravascular catheter-related infections [2] leave this aspect unanswered and categorized it as unresolved issue. Therefore we investigated microbiologically if the complete infusion line may be contaminated after short-time antibiotic and rinse-solution applications. However the issue was limited to short infusions of antibiotics, which were infused over the same limb. A constant limb flow was ensured by intermediate rinse solutions.
Methods
The sample collection for the pilot study was performed at the 72-hourly CVS-system-change of the antibiotic short infusions from July 13, 2012 to August 7, 2012 at participating intensive care units. The detailed sequence of sampling is summarized in Table 1 [Tab. 1].
Table 1: Frequency of infusion bottle exchanges within 72 h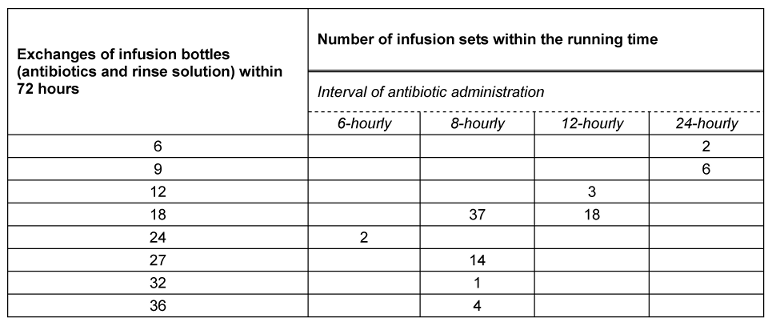
The i.v. administration sets together with the infusion bottles containing antibiotics and rinse solution (0.9% NaCl) closed with seals were packaged individually and aseptically and transported to the laboratory in a cool bag (4°C). Remaining fluid residuals were obtained aseptically from the inside lumen of i.v. administration sets inside a lamina-air-flow-bench.
To inactivate the antimicrobial activity of antibiotics residues, samples were neutralized with yolk (1 part sample solutions – 9 parts yolk suspension) from hen eggs of bio-lifestock without antibiotic feeding. Immediately before the usage, the yolk was taken with a sterile syringe suspension and mixed in the ratio of 1:1 with aqua dest. in a sterile tube. The effectiveness of the neutralization was validated in previous in-vitro dilution tests.
Approximately 15 minutes after adding the neutralizer, 0.1 mL of the solution was plated on blood agar and incubated for 48 hours at 36±1°C.
Results
Among 87 investigated samples no microbial growth was detected. One sample which hadn’t any contact to antibiotics yielded 1 colony forming unit (cfu) of coagulase-negative staphylococci (CoNS).
Within 72 hours the exchanges of the bottles differed between 6 and 36, in average 18 exchange were performed (Table 1 [Tab. 1]).
The results of our investigation suggest that i.v. administration sets which remain connected to infusion bottles (antibiotic short infusions and rinse solution) under a constant flow may be in place over duration of up to 72 hours without contamination. However for non-antibiotic flow we conclude that the i.v. administration set must be renewed at every change of an infusion bottle, because in the erroneously taken sample without any antibiotic-access 1 cfu CoNS was detected. CoNS can cause severe sepsis especially in neonates [3], [4], but they are also not harmless for adults [5], [6].
In order to interpret the results correctly it is important to note that the ICU staff who worked on the CVCs was not specifically instructed in the hygienic principles of changing an infusion bottle before the start of the pilot study. This should prevent the known effect of the training on the suppression of the central-line associated infections [7], [8], [9].
Conclusion
I.v. administration sets without any contact to antibiotics must be changed together with their infusion bottle after administration. In case of consecutive antibiotic-short- and rinse-infusions our pilot study suggests using the i.v. administration sets for up to 72 hours without renewing it at every infusion-set exchange.
However, to clarify this question into more detail, a larger consecutive study is required.
Notes
Competing interests
The study was sponsored by BBraun AG Melsungen, Germany.
References
[1] Prävention Gefäßkatheterassoziierter Infektionen. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut (RKI). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2002; 45(11): 907-24. DOI: 10.1007/s00103-002-0499-8[2] O'Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011 May;39(4 Suppl 1):S1-34. DOI: 10.1016/j.ajic.2011.01.003
[3] Huang YC, Wang YH, Su LH, Chou YH, Lien RI, Lin TY. Determining the significance of coagulase-negative staphylococci identified in cultures of paired blood specimens from neonates by species identification and strain clonality. Infect Control Hosp Epidemiol. 2006 Jan;27(1):70-3. DOI: 10.1086/499165
[4] Hira V, Sluijter M, Goessens WH, Ott A, de Groot R, Hermans PW, Kornelisse RF. Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: from population to infection. J Clin Microbiol. 2010 Nov;48(11):3876-81. DOI: 10.1128/JCM.00967-10
[5] Sharma P, Lahiri KK, Kapila K. Conventional and molecular characterization of coagulase-negative staphylococcus in hospital isolates. Indian J Pathol Microbiol. 2011 Jan-Mar;54(1):85-9. DOI: 10.4103/0377-4929.77331
[6] Fernández-Rufete A, García-Vázquez E, Hernández-Torres A, Canteras M, Ruiz J, Gómez J. Bacteriemias por Staphylococcus coagulasa negativa: análisis de factores pronóstico e influencia del tratamiento antibiótico [Coagulase-negative Staphylococcus bacteraemia: prognosis factors and influence of antibiotic treatment]. Rev Esp Quimioter. 2012 Sep;25(3):199-205.
[7] Liang SY, Khair H, Durkin MJ, Marschall J. Prevention and management of central line-associated bloodstream infections in hospital practice. Hosp Pract (Minneap). 2012 Feb;40(1):106-18. DOI: 10.3810/hp.2012.02.951
[8] Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008 Dec;36(10):S176.e1-2. DOI: 10.1016/j.ajic.2008.10.014
[9] Henderson DM, Staiger TO, Peterson GN, Sinanan MN, Angiulo CL, Makarewicz VA, Wild LM, Whimbey EE. A collaborative, systems-level approach to eliminating healthcare-associated MRSA, central-line-associated bloodstream infections, ventilator-associated pneumonia, and respiratory virus infections. J Healthc Qual. 2012 Sep-Oct;34(5):39-47; quiz 48-9. DOI: 10.1111/j.1945-1474.2012.00213.x
[10] Maki DG, Botticelli JT, LeRoy ML, Thielke TS. Prospective study of replacing administration sets for intravenous therapy at 48- vs 72-hour intervals. 72 hours is safe and cost-effective. JAMA. 1987 Oct;258(13):1777-81.




