Evaluation of synergistic effect of tazobactam with meropenem and ciprofloxacin against multi-drug resistant Acinetobacter baumannii isolated from burn patients in Tehran
Sahel Valadan Tahbaz 1Leila Azimi 2
Mahla Asadian 3
Abdolaziz Rastegar Lari 4
1 Department of Microbiology, Islamic Azad University, North Tehran Branch, Tehran, Iran
2 Pediatric Infections Research Center, Research Institute for Children’s Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3 Division of Microbiology, Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
4 Department of Microbiology, Iran University of Medical Sciences, Tehran, Iran
Abstract
Background: Acinetobacter baumannii is an increasingly important cause of nosocomial infections worldwide. In addition to the intrinsic resistance of Acinetobacter baumannii to many antibiotics, available treatment approaches with older antibiotics are significantly associated with an increase in multiresistant strains. The aim of this study was to evaluate the synergistic effect of tazobactam with meropenem and ciprofloxacin against carbapenems and drug resistant Acinetobacter baumannii isolated from burn patients in a tertiary burn center in Tehran.
Materials and methods: In this study, a total of 47 clinical isolates of A. baumannii were included from burn patients admitted to the Shahid Motahari Burns Hospital, Tehran, from June 2018 to August 2018. The disk diffusion method was used to determine resistance patterns. The synergistic effect of tazobactam with meropenem and ciprofloxacin was evaluated by determining the MIC. A PCR assay was performed to determine blaOXA-40-like, blaOXA-58-like and blaOXA-24-like.
Results: Antibiotic susceptibility testing revealed that all of the isolates were resistant to meropenem and ciprofloxacin. The MIC values decreased in the cases of combined use of ciprofloxacin and meropenem with tazobactam. The blaOXA-24-like gene was the predominant carbapenemase gene (93.6%), followed by blaOXA-40-like, which was detected in 48.9% of isolates. None of the A. baumannii isolates harbored the blaOXA-58-like gene.
Conclusions: Based on in-vitro antimicrobial susceptibility in the current study, the MIC of tazobactam combined with meropenem or ciprofloxacin have been shown to be variable. Furthermore, the data acquired from such in vitro conditions should be confirmed by reliable results from sufficiently controlled clinical trials.
Keywords
Acinetobacter baumannii, tazobactam, MIC, carbapenem resistance
Background
Burn-wound infections are considered as one of the important causes of death in developing countries [1]. Patients with severe burns are at high risk of acquiring nosocomial pathogens and contracting numerous infections as a result of the immunocompromising effects of burns, cutaneous and respiratory tract injury, prolonged hospital stays, and invasive diagnostic methods and treatment procedures [2], [3]. The control and prevention of life-threatening infectious diseases among burn patients remains a major concern worldwide, as the environment in burn units can become contaminated with resistant opportunistic pathogens [3].
Acinetobacter baumannii is considered an important nosocomially acquired opportunistic pathogen causing a wide range of severe infections, including those of burn-wounds, surgical wounds, the urinary tract (UTI), ventilator-associated pneumonia (VAP), as well as nosocomial meningitis and bacteremia [4], [5]. The bacterium is highly successful in persisting and spreading in the hospital environment, and thus can survive under dry, aharsh environmental conditions [6]. Additionally, A. baumannii can develop resistance to numerous antimicrobial agents using different mechanisms [7]. It is well documented that one of the most important factors contributing to the high mortality of A. baumannii infections is the ability to acquire a wide variety of antibiotic resistance genes and rapidly develop multidrug resistance (MDR), extensive drug resistance (XDR) and even pan-drug resistance (PDR) [8]. Dissemination of MDR A. baumannii strains has significantly limited the choice of therapeutic options available for the treatment of infections caused by this bacterium and the associated poor clinical outcome [9].
According to previously published data, carbapenems are considered as the “last-line” antibiotic against infections caused by MDR A. baumannii strains in patients and healthcare workers [10]. Due to a severely limited range of alternative therapeutic options, unfortunately, recent reports described an increasing trend of multi-drug resistance in A. baumannii in many parts of the world, so that carbapenem resistant A. baumannii strains have emerged as a major public health concern [11], [12].
However, OXA carbapenemases are significantly inhibited by clavulanic acid, sulbactam and tazobactam [13]. Thus, increasing meropenem and ciprofloxacin susceptibility in A. baumannii by considering the potential inhibitory effect of tazobactam on OXA enzymes was examined in this study.
Acinetobacter species can acquire resistance against carbapenems by producing various carbapenemase enzymes, which are members of the molecular class A, B, and D β-lactamases. The class D carbapenemases, which consist of OXA-type β-lactamases (OXA) such as blaOXA-23-like, blaOXA-24-like, blaOXA-51-like, and blaOXA-58-like, are frequently detected in MDR A. baumannii strains [14]. Although clinical use of carbapenem agents in the treatment of infections has become well established, the use of this antibiotic alone must be limited due to concerns about the emergence and spread of resistant strains. Moreover, the high mortality rates of carbapenem-resistant A. baumannii infections highlight the importance of early prediction and appropriate control measures of this bacterium in health-care settings [15]. However, little information is available on whether different treatment regimens should be used for carbapenem-resistant A. baumannii infections. Given the lack of novel antimicrobials available in the clinical setting in Iran, we investigated the effects of meropenem and ciprofloxacin alone and in combination with tazobactam on A. baumannii isolated from burn patients, in the attempt to more effectively employ available antibiotics. The aim of this study was to evaluate the synergistic effect of different concentrations of tazobactam with ciprofloxacin and meropenem, and also to detect blaOXA-24-like, blaOXA-40-like and the blaOXA-58-like genes.
Materials and methods
Sample collection and bacterial strains
The current study was carried out on 47 clinical isolates of A. baumannii obtained from patients admitted to Shahid Motahari Burns Hospital, Tehran, in a two-month period from June 2018 to August 2018. The study protocol was approved by the Ethics Committee of the National Institutes for Medical Research Development (IR NIMAD REC 1396 223), Tehran, Iran. Strains were identified by conventional biochemical and microbiological methods, e.g. oxidase, TSI, SIM, etc. In addition, to confirm A. baumannii identification, amplification and sequencing of intrinsic blaOXA-51-like genes were carried out using specific primers, as previously described [16]. All strains were stored in Tryptic Soy Broth (TSB; Merck, Germany) containing 20% glycerol at –80°C for further analysis.
Antibiotic susceptibility testing
In-vitro susceptibility testing was performed using a panel of three antibiotics in the Kirby-Bauer disc diffusion method, according to the Clinical and Laboratory Standards Institute (CLSI 2018) [17] guidelines. The antimicrobial drugs tested included imipenem (10 µg), meropenem (10 µg) and ciprofloxacin (5 µg).
Escherichia coli ATCC 25922 were used as a quality control strain in every test run. In this study, multi-drug resistance (MDR) was defined as non-sensitivity to ≥1 agent in ≥3 antimicrobial categories in CDC report [18].
Minimum inhibitory concentration (MIC) assay
The minimum inhibitory concentrations (MIC) of meropenem and ciprofloxacin were determined alone and in combination with tazobactam against the A. baumannii isolates by a macro broth dilution according to the CLSI 2018 guideline [17]. Specifically, the following concentrations were used: meropenem: 256 µg/ml to 16 µg/ml; ciprofloxacin: 128 µg/ml to 16 µg/ml. All antimicrobials were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Synergic effect of tazobactam and antibiotics assay
The minimum inhibitory concentration of each strain against meropenem and ciprofloxacin with different concentrations of tazobactam was determined. 10 µg/ml, 30 µg/ml and 50 µg/ml tazobactam were used.
Detection of carbapenem
DNA of the isolates was extracted using the boiling method as described previously [16]. The existence of class D carbapenemase genes (blaOXA-24-like, blaOXA-40-like, and blaOXA-58-like) was determined using PCR via specific primers (Table 1 [Tab. 1]). The PCR products were detected by agarose gel electrophoresis (1.5%), then they were stained with ethidium bromide and visualized under UV light (UVItec, Cambridge, UK).
Table 1: Oligonucleotide primers used in this study
Results
The results of the Kirby-Bauer disc diffusion test indicated that all of the tested isolates were resistant to meropenem, imipenem and ciprofloxacin. Therefore, all isolates were considered MDR and carbapenem-resistant A. baumannii.
Table 2 [Tab. 2] shows the MICs (µg/mL) and the susceptibility ratios of the MDR and carbapenem-resistant A. baumannii isolates for meropenem and ciprofloxacin alone and in combination with tazobactam. The MICs exhibited manifold decreases between 10 µg/mL and 30 µg/mL with 50 µg/mL in the cases of combination use of ciprofloxacin and meropenem with tazobactam. In some cases, the results showed that more than one fold reduction in compare with 50 µg/mL and 10 µg/mL, although using tazobactam alone for A. baumannii had no inhibitory effect, and all isolates grew.
Table 2: MIC intervals and susceptibility ratios against A. baumannii isolated from burn patients hospitalized in Shahid Motahari Burns Hospital, Tehran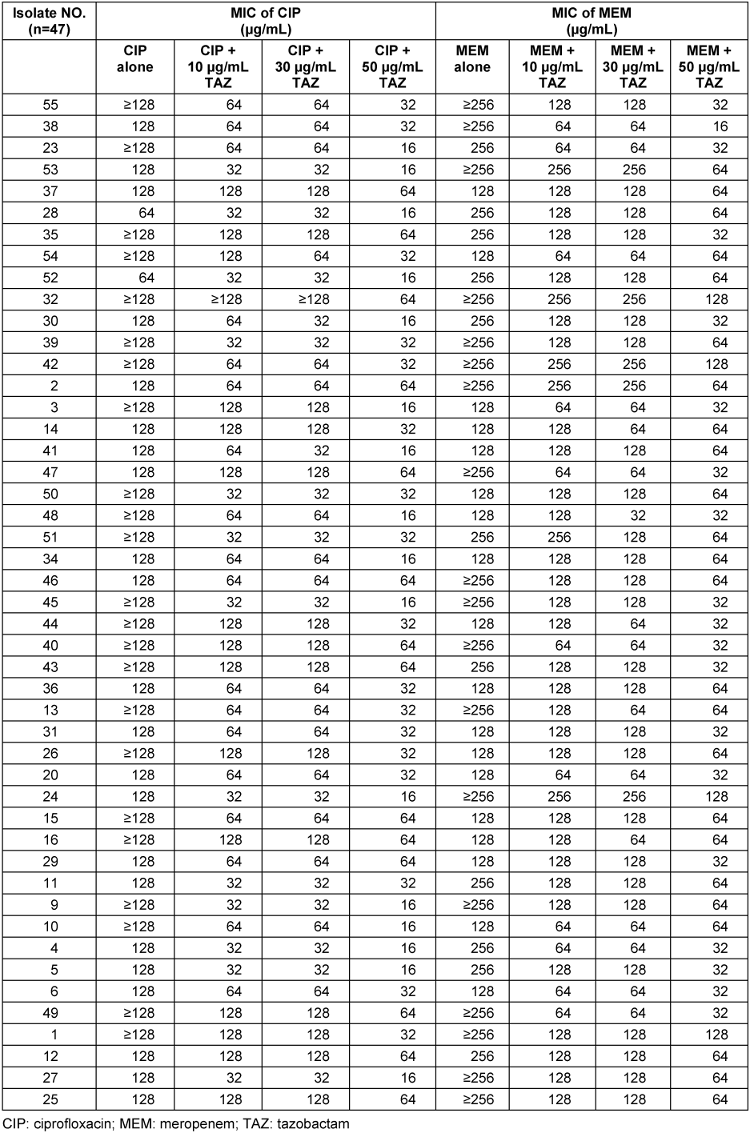
According to the results of the present study, blaOXA-24-like was the predominant carbapenemase gene (93.6%), followed by blaOXA-40-like, which was detected in 48.9% of isolates. None of the A. baumannii isolates harbored the blaOXA-58-like gene (Figure 1 [Fig. 1]). Furthermore, the co-existence of blaOXA-24-like/blaOXA-40-like was detected in 48.9% of A. baumannii isolates.
Figure 1: Frequency of resistance genes among A. baumannii isolated from burn patients hospitalized in Shahid Motahari Burns Hospital, Tehran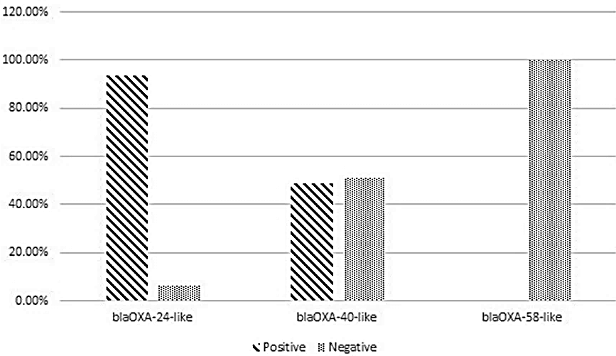
Discussion
In recent decades, the emergence of MDR and carbapenem-resistant A. baumannii isolates with a high potential for acquiring resistance to various antibiotics has been described in health settings worldwide [12], [19]. Our results indicated that all A. baumannii isolates were MDR and carbapenem resistant. The high prevalence of MDR A. baumannii strains is in accordance with the findings reported by Farsiani et al. (97%) and Rynga et al. (85%) in Iran and India, respectively [20], [21]. The global spread of MDR clones in healthcare settings has raised a great deal of concern, because carbapenem agents are commonly the first choice in the treatment of A. baumannii infections [22], [23]. The high prevalence of MDR and carbapenem-resistant A. baumannii can be attributed to the indiscriminate use of antibiotics and poor implementation of measures.
The spread of these resistant strains has impeded the successful treatment of A. baumannii infections, thus necessitating alternative treatment approaches. Among the recommended approaches, the use of a combination of antibiotics is currently the preferred treatment strategy [24]. Combination therapy is principally used to avoid the development of antimicrobial resistance, treat polymicrobial infections, and decrease dose-dependent side effects. Moreover, it is also used to treat severe infectious diseases with high mortality rates, as a combination of antimicrobial agents provides a synergistic effect against the multi-drug-resistant isolates [25]. However, the absence of antagonistic interaction among antibiotics in cases of combination therapy has clinical importance; thus, many studies have emphasized the need to determine the interactive effects of antibiotic combinations in vitro [26]. It has been previously described that the combined administration of aminoglycoside and carbapenem agents, which are the most frequently used combination in the empiric treatment of Acinetobacter infections, generally demonstrates an in vitro synergistic effect [27].
The present study attempted to investigate the in vitro interactions between tazobactam and two antibiotics, meropenem and ciprofloxacin, as possible treatment options given carbapenem-resistant A. baumannii isolates from burn patients. Although sulbactam alone has verified antibacterial activity against A. baumannii and has intrinsic bactericidal activity against MDR A. baumannii as it inhibits the penicillin- binding proteins, there are no well-documented clinical practice guidelines for tazobactam and clavulanate [26]. Tazobactam has long been used in combination with ampicillin and piperacillin, and an additive effect against clinical isolates of A. baumannii was recently observed when tazobactam was combined with meropenem or colistin [28]. However, in this study, a significant reduction in MIC was observed for meropenem when combined with tazobactam. Moreover, the in vitro efficacy of ciprofloxacin/tazobactam combinations was evaluated against A. baumannii isolates. Our findings revealed a significant reduction in MIC when ciprofloxacin and meropenem were combined with tazobactam. These results are in accordance with data reported by several authors when sulbactam was combined with amikacin and ciprofloxacin [26], [29], [30].
Our finding is in accordance with the study by Rezaei et al. in 2018 in Isfahan that indicated blaOXA-51-like was present in all strains [31]. Moreover, Mohammadi et al. reported similar results among hospitalized patients with burn infection in 2016 in Iran [32]. Therefore, it is not surprising that Chen et al. in 2017 in China, Uwingabiye et al. in 2017 in Morocco, and Nowak et al. in 2017 in Greece, Italy, and Spain reported similar results in their investigations [33], [34], [35]. The percentage of blaOXA-24-like genes, which encode acquired carbapenemases, was 93.6% in the present study, followed by blaOXA-40-like with 48.9%. Furthermore, blaOXA-58-like was not detected in our study. Accordingly, in a study in Iran, the percentage of the blaOXA-24-like gene among tested isolates was 62.1% and the blaOXA-58-like was not detected among the isolates in that study [31]. In contrast to our results, Taherikalani et al., Salehi et al., and Sohrabi et al. reported the percentage of blaOXA-58-like to be 21.2%, 11.2%, and 3.2%, respectively [36], [12], [37]. Additionally, other studies in Turkey, China, Brazil, and France indicated the presence of the blaOXA-58-like gene in A. baumannii isolates [38], [39], [40], [41].
The results of the present study demonstrated that the co-existence of blaOXA-24-like/blaOXA-40-like in half of the A. baumannii isolates. In this regard, our results and those of others confirmed that the presence of multiple alleles of the blaOXA gene or a combination of them can be directly related to the reduction of the sensitivity or resistance to some antibiotics [42], [43].
Conclusions
The results of this first study in Tehran demonstrate a high level of MDR and carbapenem-resistant A. baumannii isolates from burn patients. From a molecular standpoint, the existence of class D carbapenemase genes was established among a majority of the A. baumannii strains. Based on in vitro antimicrobial susceptibility in the current study, the MICs of tazobactam combined with meropenem or ciprofloxacin have been shown to be variable. Given the different mechanisms of antibiotic resistance in clinical isolates of A. baumannii, all results observed with a given combination is expected among A. baumannii strains. Furthermore, the data acquired from such in vitro conditions should be confirmed by reliable results from sufficiently controlled clinical trials. Because previous studies confirmed the inhibitory effect of tazobactam on OXA enzymes, the synergistic effect of tazobactam with ciprofloxacin and meropenem reflected in decreased MIC may be held responsible for inhibiting the identified OXA enzymes in the tested bacteria. In this study, also bacterial MIC was in antibiotic resistance range, so several mechanisms may be involved in the emergence of these resistances. Further investigation is necessary.
Notes
Competing interests
The authors declare that they have no competing interests.
Funding
The research reported in this publication was supported by the Elite Researcher Grant Committee under award number [IR NIMAD REC 1396 223] from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
References
[1] Tekin R, Dal T, Bozkurt F, Deveci O, Palanc Y, Arslan E, Selçuk CT, Hoşoğlu S. Risk factors for nosocomial burn wound infection caused by multidrug resistant Acinetobacter baumannii. J Burn Care Res. 2014 Jan-Feb;35(1):e73-80. DOI: 10.1097/BCR.0b013e31828a493f[2] Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006 Apr;19(2):403-34. DOI: 10.1128/CMR.19.2.403-434.2006
[3] Coban YK. Infection control in severely burned patients. World J Crit Care Med. 2012 Aug;1(4):94-101. DOI: 10.5492/wjccm.v1.i4.94
[4] An JH, Kim YH, Moon JE, Jeong JH, Kim SH, Kang SJ, Park KH, Jung SI, Jang HC. Active surveillance for carbapenem-resistant Acinetobacter baumannii in a medical intensive care unit: Can it predict and reduce subsequent infections and the use of colistin? Am J Infect Control. 2017 Jun;45(6):667-72. DOI: 10.1016/j.ajic.2017.01.016
[5] Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006 Mar;42(5):692-9. DOI: 10.1086/500202
[6] Chagas TP, Carvalho KR, de Oliveira Santos IC, Carvalho-Assef AP, Asensi MD. Characterization of carbapenem-resistant Acinetobacter baumannii in Brazil (2008-2011): countrywide spread of OXA-23-producing clones (CC15 and CC79). Diagn Microbiol Infect Dis. 2014 Aug;79(4):468-72. DOI: 10.1016/j.diagmicrobio.2014.03.006
[7] Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiol Spectr. 2016 04;4(2):. DOI: 10.1128/microbiolspec.VMBF-0016-2015
[8] Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015 Apr;40(4):277-83.
[9] Manchanda V, Sanchaita S, Singh N. Multidrug resistant acinetobacter. J Glob Infect Dis. 2010 Sep;2(3):291-304. DOI: 10.4103/0974-777X.68538
[10] Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011 Nov;55(11):4943-60. DOI: 10.1128/AAC.00296-11
[11] Codjoe FS, Donkor ES. Carbapenem Resistance: A Review. Med Sci (Basel). 2017 Dec 21;6(1):1. DOI: 10.3390/medsci6010001
[12] Salehi B, Goudarzi H, Nikmanesh B, Houri H, Alavi-Moghaddam M, Ghalavand Z. Emergence and characterization of nosocomial multidrug-resistant and extensively drug-resistant Acinetobacter baumannii isolates in Tehran, Iran. J Infect Chemother. 2018 Jul;24(7):515-523. DOI: 10.1016/j.jiac.2018.02.009
[13] Sahuquillo-Arce J, Hernández-Cabezas A, Yarad-Auad F, Ibáñez-Martínez E, Falomir-Salcedo P, Ruiz-Gaitán A. Carbapenemases: A worldwide threat to antimicrobial therapy. World J Pharmacol. 2015;4(1):75-95. DOI: 10.5497/wjp.v4.i1.75
[14] Hasan B, Perveen K, Olsen B, Zahra R. Emergence of carbapenem-resistant Acinetobacter baumannii in hospitals in Pakistan. J Med Microbiol. 2014 Jan;63(Pt 1):50-5. DOI: 10.1099/jmm.0.063925-0
[15] Lin MF, Lan CY. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J Clin Cases. 2014 Dec;2(12):787-814. DOI: 10.12998/wjcc.v2.i12.787
[16] Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SG, Livermore DM. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006 Apr;27(4):351-3. DOI: 10.1016/j.ijantimicag.2006.01.004
[17] Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: nineteenth informational supplement M100-S21. 2018.
[18] Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012 Mar;18(3):268-81. DOI: 10.1111/j.1469-0691.2011.03570.x
[19] Sirijatuphat R, Thamlikitkul V. Preliminary study of colistin versus colistin plus fosfomycin for treatment of carbapenem-resistant Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2014 Sep;58(9):5598-601. DOI: 10.1128/AAC.02435-13
[20] Farsiani H, Mosavat A, Soleimanpour S, Nasab MN, Salimizand H, Jamehdar SA, Ghazvini K, Aryan E, Baghani AA. Limited genetic diversity and extensive antimicrobial resistance in clinical isolates of Acinetobacter baumannii in north-east Iran. J Med Microbiol. 2015 Jul;64(7):767-73. DOI: 10.1099/jmm.0.000090
[21] Rynga D, Shariff M, Deb M. Phenotypic and molecular characterization of clinical isolates of Acinetobacter baumannii isolated from Delhi, India. Ann Clin Microbiol Antimicrob. 2015 Sep;14:40. DOI: 10.1186/s12941-015-0101-5
[22] Douraghi M, Jasemi S, Kodori M, Rahbar M, Boroumand MA. Evidence of Interruption of the comM Gene in a Large Series of Clinical Isolates of Multidrug-Resistant Acinetobacter baumannii. J Mol Microbiol Biotechnol. 2016;26(6):410-413. DOI: 10.1159/000448785
[23] Viehman JA, Nguyen MH, Doi Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs. 2014 Aug;74(12):1315-33. DOI: 10.1007/s40265-014-0267-8
[24] Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005 May;40(9):1333-41. DOI: 10.1086/429323
[25] Kiffer CR, Sampaio JL, Sinto S, Oplustil CP, Koga PC, Arruda AC, Turner PJ, Mendes C. In vitro synergy test of meropenem and sulbactam against clinical isolates of Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2005 Aug;52(4):317-22. DOI: 10.1016/j.diagmicrobio.2005.03.003
[26] Temocin F, Erdinc FS, Tulek N, Demirelli M, Ertem G, Kinikli S, Koksal E. Synergistic effects of sulbactam in multi-drug-resistant Acinetobacter baumannii. Braz J Microbiol. 2015 Oct-Dec;46(4):1119-24. DOI: 10.1590/S1517-838246420140101
[27] Jung SY, Lee SH, Lee SY, Yang S, Noh H, Chung EK, Lee JI. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and Bayesian network meta-analysis. Crit Care. 2017 Dec;21(1):319. DOI: 10.1186/s13054-017-1916-6
[28] Marie MA, Krishnappa LG, Alzahrani AJ, Mubaraki MA, Alyousef AA. A prospective evaluation of synergistic effect of sulbactam and tazobactam combination with meropenem or colistin against multidrug resistant Acinetobacter baumannii. Bosn J Basic Med Sci. 2015 Oct;15(4):24-9. DOI: 10.17305/bjbms.2015.526
[29] Turton JF, Ward ME, Woodford N, Kaufmann ME, Pike R, Livermore DM, Pitt TL. The role of ISAba1 in expression of OXA carbapenemase genes in Acinetobacter baumannii. FEMS Microbiol Lett. 2006 May;258(1):72-7. DOI: 10.1111/j.1574-6968.2006.00195.x
[30] Walther-Rasmussen J, Høiby N. OXA-type carbapenemases. J Antimicrob Chemother. 2006 Mar;57(3):373-83. DOI: 10.1093/jac/dki482
[31] Rezaei A, Fazeli H, Moghadampour M, Halaji M, Faghri J. Determination of antibiotic resistance pattern and prevalence of OXA-type carbapenemases among Acinetobacter baumannii clinical isolates from inpatients in Isfahan, central Iran. Infez Med. 2018 Mar;26(1):61-66.
[32] Mohammadi F, Goudarzi H, Hashemi A, Nojookambari NY, Khoshnood S, Sabzehali F. Detection of ISAba1 in Acinetobacter baumannii strains carrying OXA genes isolated from iranian burns patients. Arch Ped Infect Dis. 2017 April;5(2):e39307.
[33] Chen Y, Gao J, Zhang H, Ying C. Spread of the bla(OXA-23)-Containing Tn2008 in Carbapenem-Resistant Acinetobacter baumannii Isolates Grouped in CC92 from China. Front Microbiol. 2017 Feb 6;8:163. DOI: 10.3389/fmicb.2017.00163
[34] Uwingabiye J, Lemnouer A, Roca I, Alouane T, Frikh M, Belefquih B, Bssaibis F, Maleb A, Benlahlou Y, Kassouati J, Doghmi N, Bait A, Haimeur C, Louzi L, Ibrahimi A, Vila J, Elouennass M. Clonal diversity and detection of carbapenem resistance encoding genes among multidrug-resistant isolates recovered from patients and environment in two intensive care units in a Moroccan hospital. Antimicrob Resist Infect Control. 2017;6:99. DOI: 10.1186/s13756-017-0262-4
[35] Nowak J, Zander E, Stefanik D, Higgins PG, Roca I, Vila J, McConnell MJ, Cisneros JM, Seifert H; MagicBullet Working Group WP4. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother. 2017 Dec;72(12):3277-82. DOI: 10.1093/jac/dkx322
[36] Taherikalani M, Fatolahzadeh B, Emaneini M, Soroush S, Feizabadi MM. Distribution of different carbapenem resistant clones of Acinetobacter baumannii in Tehran hospitals. New Microbiol. 2009 Jul;32(3):265-71.
[37] Sohrabi N, Farajnia S, Akhi MT, Nahaei MR, Naghili B, Peymani A, Amiri Z, Rezaee MA, Saeedi N. Prevalence of OXA-type β-lactamases among Acinetobacter baumannii isolates from Northwest of Iran. Microb Drug Resist. 2012 Aug;18(4):385-9. DOI: 10.1089/mdr.2011.0077
[38] Deveci Ö, Dal T, Tekin R, Bozkurt F, Tekin A, Dayan S. Carbapenem resistance in Acinetobacter baumannii: where is it heading? Infez Med. 2013 Sep;21(3):211-5. DOI: 0.5455/medscience.2014.03.8124
[39] Fu Y, Jiang J, Zhou H, Jiang Y, Fu Y, Yu Y, Zhou J. Characterization of a novel plasmid type and various genetic contexts of bla OXA-58 in Acinetobacter spp. from multiple cities in China. PLoS ONE. 2014;9(1):e84680. DOI: 10.1371/journal.pone.0084680
[40] de Souza Gusatti C, Bertholdo LM, Otton LM, Marchetti DP, Ferreira AE, Corção G. First occurrence of bla OXA-58 in Acinetobacter baumannii isolated from a clinical sample in Southern Brazil. Braz J Microbiol. 2012 Jan;43(1):243-6. DOI: 10.1590/S1517-838220120001000027
[41] Barnaud G, Zihoune N, Ricard JD, Hippeaux MC, Eveillard M, Dreyfuss D, Branger C. Two sequential outbreaks caused by multidrug-resistant Acinetobacter baumannii isolates producing OXA-58 or OXA-72 oxacillinase in an intensive care unit in France. J Hosp Infect. 2010 Dec;76(4):358-60. DOI: 10.1016/j.jhin.2010.05.026
[42] Marqué S, Poirel L, Héritier C, Brisse S, Blasco MD, Filip R, Coman G, Naas T, Nordmann P. Regional occurrence of plasmid-mediated carbapenem-hydrolyzing oxacillinase OXA-58 in Acinetobacter spp. in Europe. J Clin Microbiol. 2005 Sep;43(9):4885-8. DOI: 10.1128/JCM.43.9.4885-4888.2005
[43] Jeon BC, Jeong SH, Bae IK, Kwon SB, Lee K, Young D, Lee JH, Song JS, Lee SH. Investigation of a nosocomial outbreak of imipenem-resistant Acinetobacter baumannii producing the OXA-23 beta-lactamase in korea. J Clin Microbiol. 2005 May;43(5):2241-5. DOI: 10.1128/JCM.43.5.2241-2245.2005
[44] Héritier C, Poirel L, Lambert T, Nordmann P. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2005 Aug;49(8):3198-202. DOI: 10.1128/AAC.49.8.3198-3202.2005




