Prevalence of methicillin sensitive and resistant Staphylococcus aureus carriage among German emergency medical providers
Aaron Weiss 1Axel Kramer 1
Robert Taube 2
Frauke Mattner 3
Katrin Premke 4
1 Institute of Hygiene and Environmental Medicine, University Medicine Greifswald, Greifswald, Germany
2 Technical Microbiology, Faculty of Nature and Engineering, City University of Applied Sciences, Bremen, Germany
3 Institute for Hygiene, Cologne Merheim Medical Centre, University Hospital Witten/Herdecke, Germany
4 Department of Tropical Medicine and Infectious Diseases, Rostock University Medical Center, Rostock, Germany
Abstract
Background: Health care workers (HCW) in Emergency Medical Services (EMS) frequently come into contact with carriers of methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) strains and may acquire and transmit them to patients. However, there is little data on MSSA and MRSA colonization of medical personnel in the emergency services. Additionally, few studies have analyzed the association between personal hygiene of staff and colonization. Therefore, we examined the prevalence of MSSA and MRSA in EMS staff of two German regions and evaluated their personal hygiene behavior.
Method: Throat and nasal swabs from 300 EMS workers were analyzed. Both direct and pre-enriched cultures of the swabs were cultivated on culture media to identify MSSA and MRSA. Results were analyzed together with questionnaires about sociodemographic data and a self-assessment of hygiene behavior. Statistical analysis was done using the R statistical software.
Results: Of the total 300 swabs, 55% were from paramedics, 39% were from emergency medical technicians (EMT) and 5% were from emergency physicians. With 1%, the MRSA prevalence was comparable to that of the German population, while the MSSA rate – 43.7% – was higher than expected. Colonization with MSSA was significantly associated with poor hand hygiene and male sex, and was inversely correlated to time on the job in EMS.
Conclusion: The sample size of 300 and a MRSA prevalence of 1% made a meaningful analysis of potential influencing factors on the prevalence of MRSA infeasible. The comparatively high prevalence of MSSA and the association with decreasing frequency of hand antisepsis suggests an influence of personal hygiene on MSSA colonization. HCW in EMS should be encouraged to make use of their personal protective equipment and practice frequent hand hygiene. The implementation of diagnostic tools such as the Hand Hygiene Self-Assessment Framework of the WHO could be utilized to reveal problems in organizations, followed by an individual program to promote hand hygiene.
Keywords
S. aureus carrier, MRSA carrier, nasal and throat carriage, emergency medical services, personal hygiene
Introduction
Methicillin sensitive Staphylococcus aureus (MSSA) is an opportunistic pathogen which can colonize the upper respiratory tract. Cross-sectional studies have found an MSSA colonization rate of 20–30% of the population worldwide [1], [2] and 22–41% in the German population [3], [4], [5]. Compared to MSSA, colonization with MRSA is less common. with a prevalence ranging from 0.3–1.3% in the German population [3], [4]. Colonized humans are asymptomatic, with symptoms arising only in the event of infection. Thus, both MSSA and MRSA could unknowingly be spread within communities. Despite the low prevalence of MRSA in Germany, colonization with MRSA facilitates transmission and predisposes to various invasive infections [6], [7]. Both community acquired and nosocomial MRSA infections cause financial burdens [8], [9] and are responsible for an increased mortality [10]. The spread of MRSA is facilitated by direct contact to patients or healthcare workers (HCW) colonized or infected with MRSA [11], and can occur via airborne transmission in individuals with nasal carriage [12] as well as through contact to contaminated objects and surfaces on which MRSA can persist for months [13]. To minimize its spread, data on the frequency of MRSA carriage in HCW is needed. As emergency medical service (EMS) personnel could well play a unique role in transmission by linking the general population to high risk environments such as intensive care units (ICU), emergency departments (ED) and nursing homes [14], surveillance in this sector is important. Although there is a paucity of data on the prevalence of MRSA carriage in EMS [15], [16], it has been shown to vary from 0.7% to 21.2% [15], [16], [17], [18]. To reveal areas of high prevalence and contain transmission, local surveillance appears necessary. Furthermore, by evaluating the carrier rate of MRSA and MSSA in EMS personnel and comparing it to the general population, the efficacy of current infection control concepts can be assessed. It may be hypothesized that if the carrier rate in EMS, despite the higher risk of exposure [15], [18], is not significantly higher than the carrier rate of the general public, the protective measures in place are effective; this has already been confirmed in dental healthcare workers [19]. Thus, the aim of this study was to identify the prevalence of MSSA and MRSA and evaluate current infection control concepts of EMS in two geographically distant regions of Germany.
Materials and methods
Study design and recruitment
The study performed in two different regions of Germany, Cologne and Greifswald. Throat and nasal swabs were taken from 304 EMS workers, which included emergency physicians (EP), emergency medical technicians (EMT) and paramedics from both fire departments and private EMS companies. Inclusion criteria were a minimum age of 18 years, voluntary written consent, a minimum work experience of one year and average weekly working time in EMS of at least 12 hours. Due to missing questionnaires, 300 swabs were analyzed. It is noteworthy that samples in the second cohort (Greifswald) were taken from 08.12.2020 to 30.06.2021 during peaks of the COVID-19 pandemic, while samples from the Cologne group were taken from 26.06.2019 to 07.10.2019.
Sample collection and supporting questionnaire
To ensure proper sampling and minimize subjective error, the same person – who was instructed and trained in the swabbing technique – took samples from participants. A single swab was used to take samples from the posterior pharynx as well as from both anterior nares.
Additionally, participants filled out a questionnaire about demographics, weekly working hours, and years of experience. The questionnaire also contained items on self-assessment of the participants’ own hygiene behavior during different scenarios at work, treatment with antibiotics within the last two months, and the presence of immunodeficiency. Furthermore, contact to groups at risk for MSSA/MRSA colonization within the preceding six months were queried. Participants had to indicate whether they consciously had direct contact to those groups, e.g., in patient care/transport, or only indirect contact via co-workers or partners who cared for patients in the respective groups. Participants who stated that they had neither direct nor indirect contact were placed in the category of indirect contact, as we found it extremely unlikely that workers in EMS have no contact whatsoever with the groups in question. The complete questionnaire can be found in the supplements.
Sample processing
Analysis of specimens was started within 48 hours after they were obtained. Swabs were transferred promptly to either the central laboratory of the Cologne-Merheim Clinic or the University Hospital Greifswald. Samples in the Cologne group were obtained from 26.06.2019 to 07.10.2019 using the Copan eSwab® system (Copan, Italy). Samples from Greifswald were taken from 08.12.2020 to 30.6.2021 using Transystem® with Amies agar gel medium (HAIN Lifescience, Nehren, Germany) [19]. Both direct and pre-enriched cultures were cultivated. Samples from the Cologne group were streaked onto Columbia CNA agar with 5% sheep blood to identify MSSA, while in the Greifswald group, mannitol salt agar (BD GmbH, Heidelberg, Germany) for MSSA was used. CHROMagar™ (BD GmbH, Heidelberg, Germany) was used in both groups to test for MRSA.
A detailed description of the methods can be found in the supplements.
Data preparation
Persons with missing answers or the answer “unsure” regarding contact to risk groups were excluded from the statistical analysis. The variables “soft tissue defects or chronic wounds” and “atopic dermatitis” were not analyzed, as they had a high number of missing answers which would have led to a drastic reduction of our datasets. After exclusion of those subjects, 262 datasets remained for analysis. Contact to persons working on ICU and patients receiving mechanical ventilation were placed in the cluster “ICU2”.
The cluster “Nursing” contained individuals working in outpatient care, individuals chronically in need of care, and individuals in long-term care facilities.
Statistical analysis
Statistical analyses were performed with R software (version 4.1.3, R Foundation, Vienna, Austria). Statistical significance was set at p<0.05. The Chi-squared goodness-of-fit test was applied to compare the observed frequencies of infected and non-infected individuals to previous studies. Fisher’s exact test was employed to analyze pairwise dependence between variables. The Bonferroni method was used to correct for multiple comparisons. The Wilcoxon rank-sum test was performed to analyze differences between two groups. Spearman’s correlation was used to analyze associations between variables with multiple levels. Multivariate logistic regression models (binomial distribution) were applied to analyze the dependencies of replies in the questionnaire on the state of colonization. Backward selection based on Akaike’s information criterion was conducted to select predictive variables. The final model was cross-validated with the caret package (version 6.0-9.4), using 20% of the dataset as test data with 100 resamplings.
Results
Baseline data
Out of 305 enrolled participants, 304 nasal swabs could be analyzed, 300 persons filled out the questionnaire and 262 datasets remained for analysis after adjustment of data.
Participants from Greifswald were on average 7 years older than participants from Cologne (30 vs. 37 years, on average), with more people working 48-hour weeks (64.4% vs 45.2%, on average), and more experienced personnel, with 37.3% working >10 years in EMS compared to 21.2% in Cologne (Table 1 [Tab. 1]). Both groups contained four times more male (79%) than female participants (21%). Most participants were paramedics (55%), followed by emergency medical technicians (39%) and emergency physicians (5%). In terms of age distribution by professional group, physicians were mostly older persons, most paramedics were in their mid-20s to mid-30s, and EMTs tended to be under 25 years of age. In total, 60% of the participants had work experience of 6–25 years and worked on average 48 hours a week. Most of the participants were Caucasian (78%), 2% were Asian and 20% chose “other” or provided no information about their ethnicity.
Table 1: Sociodemographic differences in prevalence of MRSA and MSSA samples in Greifswald and Cologne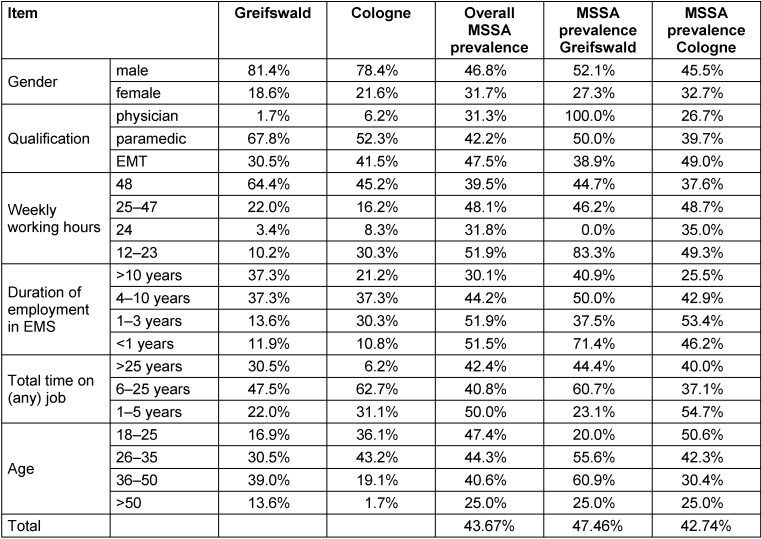
MSSA and MRSA
Almost half of the total samples were positive for MSSA (43.7%) and 3 (1%) were MRSA-positive. MRSA carriage only occurred in the participants from Cologne, with the positively screened persons being distributed across the three professional groups.
Hygiene behavior
Table 2 [Tab. 2] shows data of the participants’ self-assessment regarding their personal hygiene behavior. 83.3% of participants stated they performed hand antisepsis after every treatment; 85% reported glove use during every treatment. Use of facemasks, gowns and goggles differed between the two cohorts, with participants from Greifswald displaying more frequent use of this personal protective equipment (PPE). The most pronounced difference between the two cohorts was the use of facemasks: in Cologne, 0.4% stated wearing one during every call, but in Greifswald, 89.8% of the participants claimed they used facemasks for each treatment (p<0.0001). Hygiene behavior showed some statistically significant differences according to duration of employment in EMS. Participants with longer employment in EMS showed a reduced use of gloves (ρ=–0.21, p.adj=0.0006), but a significantly increased use of facemasks (ρ=0.16, p.adj=0.009), while hand antisepsis remained similar (ρ=–0.02, p.adj= 0.71).
Table 2: Data of the self-assessment of participants’ hygiene behavior during work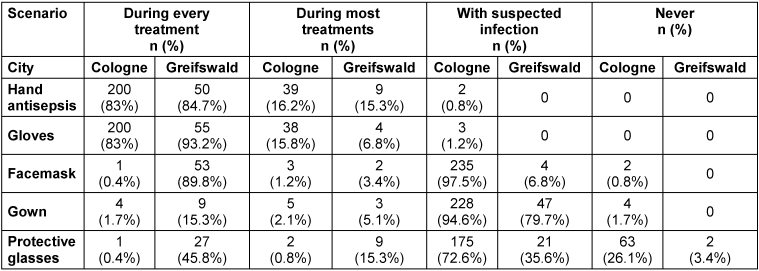
Probability of colonization with MSSA
Through backward selection, we identified several variables that predicted MSSA colonization (accuracy after cross validation=0.64; McFadden R2=0.24). Gender, duration of employment, hospitalization, and hand hygiene were significant predictors. Male individuals showed a significantly higher probability of being colonized with MSSA (p=0.007). Our data showed that with increasing time on the job, it became less likely for persons to be colonized with MSSA (p=0.006). Especially people who had worked in EMS for over 10 years were less often colonized with MSSA. Concerning contact to risk groups, direct contact to persons who were hospitalized for more than three days within the last twelve months was highly significantly associated with MSSA colonization (p=0.005), whereas contact to persons with immunosuppression or the ICU2-cluster did not increase the likelihood of MSSA colonization (Figure 1 [Fig. 1]). Lastly, the more often people reported practicing hand hygiene, the less often they were colonized with MSSA (p=0.019).
Figure 1: Contact of participants to groups at risk for MSSA/MRSA colonization. 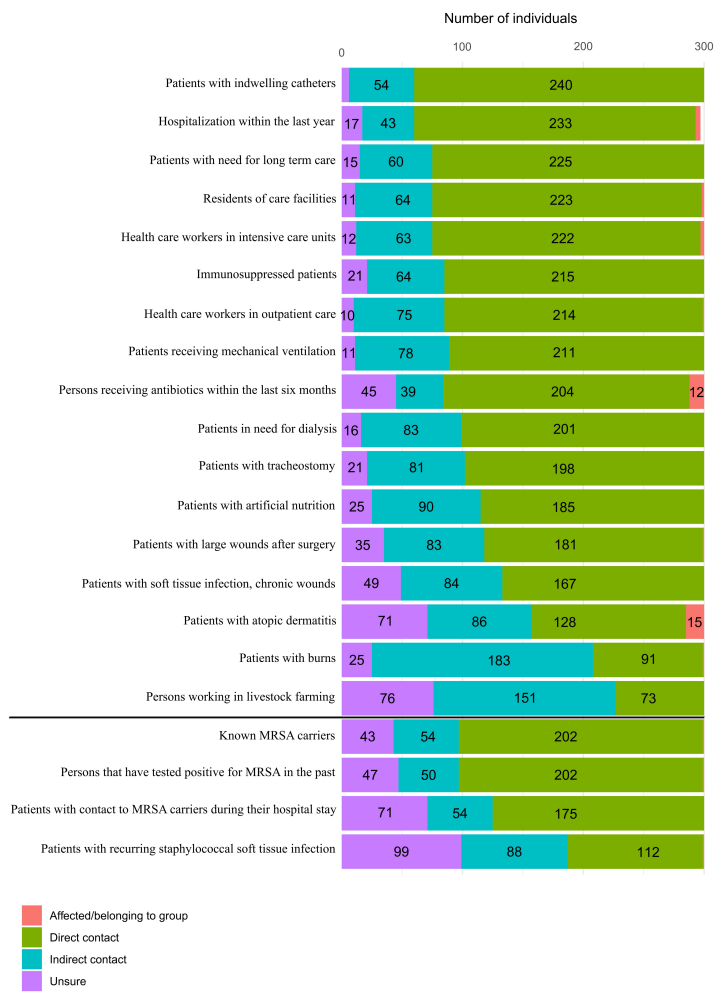
Participants had to specify whether they received antibiotics within the last two months, if they were diagnosed with an immune deficiency and whether they consciously had direct contact to the above-mentioned groups, such as in patient care/transport, or only indirect contact, such as via co-workers or partners who cared for patients in the respective groups. People stating they had neither direct nor indirect contact were placed in the category of indirect contact.
Discussion
To the best of our knowledge, this is the largest study testing for nasal/pharyngeal MRSA and MSSA carriage in EMS with additional consideration and evaluation of personal hygiene. Generally, studies on nasal S. aureus carriage in EMS are rare, although this profession is thought to be an important link between patients, hospitals and multidisciplinary staff [14].
Method
Studies examining MRSA prevalence in EMS often test for MRSA using swabs of the anterior nares [15], [16]. Although traditionally thought to be the most important site to test for S. aureus colonization [7], The sensitivity was shown to be as low as 48% [20] to 66% [21] when testing the nasal vestibule alone. We used one swab to test both the pharynx and both anterior nares, which in one study increased sensitivity by 25.7% [22]. Sampling by the same investigator guarantees a high level of standardization. Furthermore, the different analytical methods in laboratories influence the sensitivity in MRSA detection. One study showed that up to 1/3 of MRSA cases were overlooked when a direct culture without enrichment was used, compared to PCR analysis of the same sample [23]. We analyzed specimens using pre-enriched cultures, which exhibited a sensitivity only slightly lower than PCR analysis while being less cost-intensive [23].
MRSA and MSSA nasal colonization in EMS
With 1%, the prevalence of MRSA does not differ significantly from MRSA prevalence in the German general population (Table 3 [Tab. 3]). Regarding other countries, the prevalence among HCW of EMS in our study is in the range of the 0.7% reported for Virginia, USA [16], but lower than the 3.2% in 2018 in Hamburg, Germany [24], 4.6% in Ohio, USA [25] or 21.2% in Portugal (Table 4 [Tab. 4], [16], [17]). These geographical differences are well-known and partly attributable to misuse of antibiotics; however, the situation is much more complex due to geographical and socioeconomic factors, such as political stability, water quality, access to health care, and implementation of infection and surveillance control mechanisms [26], [27]. MSSA, on the other hand, shows a more even distribution of nasal colonization, with 20–30% globally and 22–41% in the German population [1], [2], [3], [4]. The MSSA rate of EMS in Greifswald was 47.5%. This is twice as high as rates found in surveillance data in Northern Germany of the regional community with 21.9% [5] and 27.2% [3] and data of dental healthcare workers from Northern Germany (22.3%) [19]. This may indicate inconsistent adherence of HCW in EMS to their PPE and standard precautions. Due to the lack of surveillance data for MSSA in Cologne, the MSSA rate of 42.7% in EMS in Cologne could not be compared. However, the higher prevalence of MSSA in Greifswald than in Cologne (47.5% vs. 42.7%) was not statistically significant (p=0.72).
Table 3: MRSA prevalence in the German population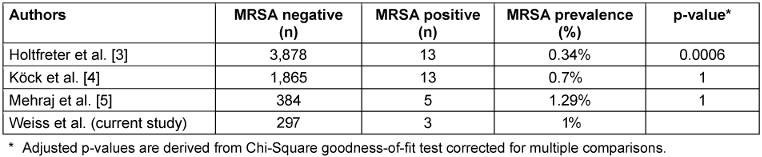
Table 4: Prevalence of MRSA and MSSA carriages in different populations EMS 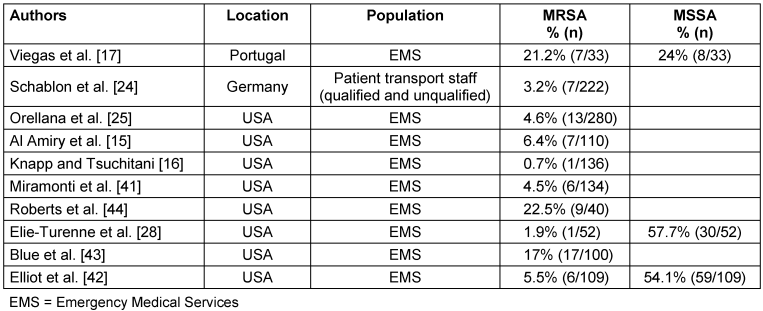
Additionally, we determined high rates of direct contact to patient populations that pose a risk for MSSA transmission (Figure 1 [Fig. 1]), as well as frequent contact with hospital environments, which could contribute to the higher rates of MSSA. Other factors, such as gender or ethnicity, may also explain the higher carrier rates in our study compared to the German general population, as it was shown that Caucasian people and men have higher rates of MSSA carriage [1]. Our study participants consisted of roughly 80% men and only 20% women, with 78% Caucasian ethnicity, which might drive up the prevalence of MSSA in this study. Most other studies in EMS differ regarding MRSA and MSSA distribution (Table 4 [Tab. 4]). Only one study showed similar distribution and prevalence, with 1.9% MRSA-positive and 57.7% MSSA-positive (p=0.134, [28]). The high heterogeneity of MSSA and especially MRSA prevalence in the different studies is worth mentioning. These differences, with their poorly traceable and likely multifactorial influences, lead to the conclusion that each health care sector (EMS, ED, ICU) must undertake their own investigations to find out whether a serious burden of MSSA and MRSA exists. In doing so, further outbreaks could be prevented and sources could be isolated.
Due to sampling during the COVID-19 pandemic in the second cohort (Greifswald), PPE use was enhanced by using FFP2 masks and safety goggles during every call without suspicion of COVID-19, and by using additional gowns/aprons around patients with suspected or confirmed COVID-19 infection. As such hygienic measures might also protect against colonization with different microorganisms, and our data show high adherence to use of PPE (Table 3 [Tab. 3]), the rate of MRSA carriage could have been reduced by this.
Distribution of nasal MRSA colonization and its limitations in evaluation
As previously stated, pronounced differences in prevalence are multifactorial, including differences in the prevalence of MRSA in the various geographic regions, local EMS protocols for hygienic measures and adherence of staff to them. Moreover, the type of study (longitudinal or cross-sectional) and testing influences results. Cross-sectional studies might fail to depict the true prevalence of MRSA, as colonization with S. aureus is not a dichotomous, but rather a dynamic event, including roughly 30% intermittent carriers which, in point-prevalence studies can either test positive or negative [1]. Although conducting longitudinal studies with a series of tests at different times can solve this problem, such studies are more difficult, complex, time consuming and cost intensive. While cross-sectional studies are not the most accurate indicator of MRSA carriage, they can provide an estimate to evaluate whether local hygiene protocols in place are effective in containing MRSA spread to protect healthcare workers and their patients.
Influence of socio-demographic characteristics on S. aureus carriage
Our data show that at the time of testing, the majority of EMS personnel in both cohorts was male (79%) and Caucasian (78%), which is in accordance with other studies [15], [25].
The significantly higher association of MSSA carriage with male gender is well recognized [3], [4], [29]. The reasons for this are manifold, including less optimal hand hygiene practiced by males [30], [31] and differences in sex hormones, which were shown to modulate immune response and interaction of host and microorganism, leaving males more susceptible to many bacterial infections [32], [33]. In both our cohorts, not older age itself but longer time in EMS was associated with statistically significant decreases in MSSA rates. Only a few studies investigating MSSA prevalence failed to demonstrate a significant correlation of age to MSSA carriage [5], [34], while most studies showed an inverse correlation of age with colonization status [1], [3], [29]. A possible explanation for this phenomenon could be an immunity buildup due to repeated exposure to S. aureus. Also, one could hypothesize that our study did not show significant age-related differences in MSSA carriage due to a lack of enough older participants to demonstrate the effect. Furthermore, a longer time in EMS could accelerate the natural immunity buildup due to more frequent exposure to MSSA and thus mimic the effects of older age on colonization status.
Importance of the hygiene concept in the spread of S. aureus
Implementing systems to contain the spread of pathogens and especially MDRO (multidrug-resistant organisms) is the key element in reducing healthcare-associated infections. HCW are a recognized vector in transmitting MDRO [11], [35] and often show suboptimal compliance with standard precautions such as hand hygiene [36]. Especially EMS providers were found to have extremely low adherence to standard indications for practicing hand hygiene and standard hygiene precautions [37], [38]. Our questionnaire used basic scenarios (during every treatment, during most treatments, with suspected infection, never) from which participants could choose when they practice different precautions and use of PPE. As using the term “during every treatment” does not cover the different indications for practicing hand hygiene, one can only assume that the percentage of EMS workers correctly practicing hand hygiene is even lower. Several studies show that implementation of programs to increase hand hygiene compliance are effective, can reduce MRSA cross-transmission, nosocomial-/bloodstream infections with MRSA, and are cost-effective [39], [40]. Collection of data via questionnaires can help to reveal fundamental hygiene problems within organizations, but it does not replace studies that aim to assess compliance. The results regarding adherence to hygiene measures in this study can only indicate a trend. It is also worth mentioning that despite anonymization, the self-assessment might be susceptible to social desirability bias, where the participants tend to choose answers they know are favorable. In conclusion, it might be beneficial to err on the side of caution, assume knowledge gaps and poor compliance, and implement programs to prevent as many transmissions and infections as possible.
Limitations
With a carrier rate for MRSA of 1%, the sample size of 300 does not allow any conclusions to be drawn about potential influences on colonization.
Another limitation is the possibility of selection bias, as known MRSA carriers might tend to not participate in this study, by which MRSA prevalence could be underestimated. In contrast to the low MRSA rate, the MSSA prevalence was 43.67%, which is noticeably high. This should be interpreted with the caveat that cross-sectional studies do not identify intermittent carriers, thus possibly over- or underestimating the true prevalence. Furthermore, as we used a self-assessment for hygiene behavior, the reported adherence may be overestimated.
Conclusions
With a prevalence of 1%, MRSA prevalence in EMS does not differ from that of the German population. The high MSSA prevalence, the correlation of MSSA carriage with poor hand hygiene, and the known low adherence of EMS staff to the latter lead to the conclusion that there is room for improvement regarding hygienic behavior in German EMS staff. We recommend implementing a diagnostic tool such as the Hand Hygiene Self-Assessment Framework of the WHO [36] to reveal problems in hygiene, followed by an individual program to promote hand hygiene in the respective institutions.
Notes
Authors’ ORCIDs
- Aaron Weiss: 0009-0002-7735-7182
- Axel Kramer: 0000-0003-4193-2149
- Robert Taube: 0000-0003-3136-8732
- Frauke Mattner: 0009-0008-2268-6310
- Katrin Premke: 0000-0001-6216-5386
Ethical approval
This study was conducted after approval by the ethics committee of the University of Greifswald (internal registration number BB042/19).
Funding
None.
Acknowledgments
We gratefully acknowledge the participation of the 304 EMS workers for filling out the questionnaire and for allowing us to take the swabs. The project could not have been carried out without their help. We thank Ingo Winterfeld from the Institute for Hygiene of the Cologne-Merheim Clinic for helping us set up and organize sampling and analysis in Cologne.
Competing interests
The authors declare that they have no competing interests.
References
[1] Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005 Dec;5(12):751-62. DOI: 10.1016/S1473-3099(05)70295-4[2] van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis. 2009 Jun;199(12):1820-6. DOI: 10.1086/599119
[3] Holtfreter S, Grumann D, Balau V, Barwich A, Kolata J, Goehler A, Weiss S, Holtfreter B, Bauerfeind SS, Döring P, Friebe E, Haasler N, Henselin K, Kühn K, Nowotny S, Radke D, Schulz K, Schulz SR, Trübe P, Vu CH, Walther B, Westphal S, Cuny C, Witte W, Völzke H, Grabe HJ, Kocher T, Steinmetz I, Bröker BM. Molecular Epidemiology of Staphylococcus aureus in the General Population in Northeast Germany: Results of the Study of Health in Pomerania (SHIP-TREND-0). J Clin Microbiol. 2016 Nov;54(11):2774-85. DOI: 10.1128/JCM.00312-16
[4] Köck R, Werner P, Friedrich AW, Fegeler C, Becker K; Prevalence of Multiresistant Microorganisms (PMM) Study Group; Prevalence of Multiresistant Microorganisms PMM Study Group. Persistence of nasal colonization with human pathogenic bacteria and associated antimicrobial resistance in the German general population. New Microbes New Infect. 2016 Jan;9:24-34. DOI: 10.1016/j.nmni.2015.11.004
[5] Mehraj J, Akmatov MK, Strömpl J, Gatzemeier A, Layer F, Werner G, Pieper DH, Medina E, Witte W, Pessler F, Krause G. Methicillin-sensitive and methicillin-resistant Staphylococcus aureus nasal carriage in a random sample of non-hospitalized adult population in northern Germany. PLoS One. 2014;9(9):e107937. DOI: 10.1371/journal.pone.0107937
[6] von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med. 2001 Jan;344(1):11-6. DOI: 10.1056/NEJM200101043440102
[7] Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998 Aug;339(8):520-32. DOI: 10.1056/NEJM199808203390806
[8] Klein EY, Jiang W, Mojica N, Tseng KK, McNeill R, Cosgrove SE, Perl TM. National Costs Associated With Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus Hospitalizations in the United States, 2010-2014. Clin Infect Dis. 2019 Jan;68(1):22-8. DOI: 10.1093/cid/ciy399
[9] Hübner C, Hübner NO, Hopert K, Maletzki S, Flessa S. Analysis of MRSA-attributed costs of hospitalized patients in Germany. Eur J Clin Microbiol Infect Dis. 2014 Oct;33(10):1817-22. DOI: 10.1007/s10096-014-2131-x
[10] Bai AD, Lo CKL, Komorowski AS, Suresh M, Guo K, Garg A, Tandon P, Senecal J, Del Corpo O, Stefanova I, Fogarty C, Butler-Laporte G, McDonald EG, Cheng MP, Morris AM, Loeb M, Lee TC. Staphylococcus aureus bacteraemia mortality: a systematic review and meta-analysis. Clin Microbiol Infect. 2022 Aug;28(8):1076-84. DOI: 10.1016/j.cmi.2022.03.015
[11] Albrich WC, Harbarth S. Health-care workers: source, vector, or victim of MRSA? Lancet Infect Dis. 2008 May;8(5):289-301. DOI: 10.1016/S1473-3099(08)70097-5
[12] Sherertz RJ, Bassetti S, Bassetti-Wyss B. “Cloud” health-care workers. Emerg Infect Dis. 2001;7(2):241-4. DOI: 10.3201/eid0702.010218
[13] Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006 Aug;6:130. DOI: 10.1186/1471-2334-6-130
[14] Al Amiry A. Methicillin-resistant Staphylococcus aureus: An occupational health hazard in the prehospital setting. J Acute Dis. 2015;4:274-6. DOI: 10.1016/j.joad.2015.06.003.
[15] Al Amiry A, Bissell RA, Maguire BJ, Alves DW. Methicillin-resistant staphylococcus aureus nasal colonization prevalence among Emergency Medical Services personnel. Prehosp Disaster Med. 2013 Aug;28(4):348-52. DOI: 10.1017/S1049023X13003476
[16] Knapp BJ, Tsuchitani SN. Prevalence of methicillin-resistant Staphylococcus aureus colonization in emergency medical service providers. Am J Emerg Med. 2011 Oct;29(8):957-8. DOI: 10.1016/j.ajem.2011.05.024
[17] Viegas C, Sousa P, Dias M, Caetano LA, Ribeiro E, Carolino E, Twarużek M, Kosicki R, Viegas S. Bioburden contamination and Staphylococcus aureus colonization associated with firefighter's ambulances. Environ Res. 2021 Jun;197:111125. DOI: 10.1016/j.envres.2021.111125
[18] Sexton JD, Reynolds KA. Exposure of emergency medical responders to methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2010 Jun;38(5):368-73. DOI: 10.1016/j.ajic.2010.01.004
[19] Lerche N, Holtfreter S, Walther B, Semmler T, Al'Sholui F, Dancer SJ, Daeschlein G, Hübner NO, Bröker BM, Papke R, Kohlmann T, Baguhl R, Seifert U, Kramer A. Staphylococcus aureus nasal colonization among dental health care workers in Northern Germany (StaphDent study). Int J Med Microbiol. 2021 Aug;311(6):151524. DOI: 10.1016/j.ijmm.2021.151524
[20] Senn L, Basset P, Nahimana I, Zanetti G, Blanc DS. Which anatomical sites should be sampled for screening of methicillin-resistant Staphylococcus aureus carriage by culture or by rapid PCR test? Clin Microbiol Infect. 2012 Feb;18(2):E31-3. DOI: 10.1111/j.1469-0691.2011.03724.x
[21] Matheson A, Christie P, Stari T, Kavanagh K, Gould IM, Masterton R, Reilly JS. Nasal swab screening for methicillin-resistant Staphylococcus aureus--how well does it perform? A cross-sectional study. Infect Control Hosp Epidemiol. 2012 Aug;33(8):803-8. DOI: 10.1086/666639
[22] Mertz D, Frei R, Jaussi B, Tietz A, Stebler C, Flückiger U, Widmer AF. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis. 2007 Aug;45(4):475-7. DOI: 10.1086/520016
[23] Tsang STJ, McHugh MP, Guerendiain D, Gwynne PJ, Boyd J, Simpson AHRW, Walsh TS, Laurenson IF, Templeton KE. Underestimation of Staphylococcus aureus (MRSA and MSSA) carriage associated with standard culturing techniques: One third of carriers missed. Bone Joint Res. 2018 Jan;7(1):79-84. DOI: 10.1302/2046-3758.71.BJR-2017-0175.R1
[24] Schablon A, Kleinmüller O, Nienhaus A, Peters C. MRSA prevalence among patient transport staff in Hamburg. GMS Hyg Infect Control. 2018;13:Doc03. DOI: 10.3205/dgkh000309
[25] Orellana RC, Hoet AE, Bell C, Kelley C, Lu B, Anderson SE, Stevenson KB. Methicillin-resistant Staphylococcus aureus in Ohio EMS Providers: A Statewide Cross-sectional Study. Prehosp Emerg Care. 2016;20(2):184-90. DOI: 10.3109/10903127.2015.1076098
[26] Collignon P, Beggs JJ, Walsh TR, Gandra S, Laxminarayan R. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018 Sep;2(9):e398-e405. DOI: 10.1016/S2542-5196(18)30186-4
[27] Lee AS, de Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018 May;4:18033. DOI: 10.1038/nrdp.2018.33
[28] Elie-Turenne MC, Fernandes H, Mediavilla JR, Rosenthal M, Mathema B, Singh A, Cohen TR, Pawar KA, Shahidi H, Kreiswirth BN, Deitch EA. Prevalence and characteristics of Staphylococcus aureus colonization among healthcare professionals in an urban teaching hospital. Infect Control Hosp Epidemiol. 2010 Jun;31(6):574-80. DOI: 10.1086/652525
[29] den Heijer CD, van Bijnen EM, Paget WJ, Pringle M, Goossens H, Bruggeman CA, Schellevis FG, Stobberingh EE; APRES Study Team. Prevalence and resistance of commensal Staphylococcus aureus, including meticillin-resistant S aureus, in nine European countries: a cross-sectional study. Lancet Infect Dis. 2013 May;13(5):409-15. DOI: 10.1016/S1473-3099(13)70036-7
[30] Suen LKP, So ZYY, Yeung SKW, Lo KYK, Lam SC. Epidemiological investigation on hand hygiene knowledge and behaviour: a cross-sectional study on gender disparity. BMC Public Health. 2019 Apr;19(1):401. DOI: 10.1186/s12889-019-6705-5
[31] Anderson JL, Warren CA, Perez E, Louis RI, Phillips S, Wheeler J, Cole M, Misra R. Gender and ethnic differences in hand hygiene practices among college students. Am J Infect Control. 2008 Jun;36(5):361-8. DOI: 10.1016/j.ajic.2007.09.007
[32] Fischer J, Jung N, Robinson N, Lehmann C. Sex differences in immune responses to infectious diseases. Infection. 2015 Aug;43(4):399-403. DOI: 10.1007/s15010-015-0791-9
[33] Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000 Aug;24(6):627-38. DOI: 10.1016/s0149-7634(00)00027-0
[34] Skråmm I, Moen AE, Bukholm G. Nasal carriage of Staphylococcus aureus: frequency and molecular diversity in a randomly sampled Norwegian community population. APMIS. 2011 Aug;119(8):522-8. DOI: 10.1111/j.1600-0463.2011.02758.x
[35] Teter J, Millin MG, Bissell R. Hand hygiene in emergency medical services. Prehosp Emerg Care. 2015;19(2):313-9. DOI: 10.3109/10903127.2014.967427
[36] de Kraker MEA, Tartari E, Tomczyk S, Twyman A, Francioli LC, Cassini A, Allegranzi B, Pittet D. Implementation of hand hygiene in health-care facilities: results from the WHO Hand Hygiene Self-Assessment Framework global survey 2019. Lancet Infect Dis. 2022 Jun;22(6):835-44. DOI: 10.1016/S1473-3099(21)00618-6
[37] Vikke HS, Vittinghus S, Giebner M, Kolmos HJ, Smith K, Castrén M, Lindström V. Compliance with hand hygiene in emergency medical services: an international observational study. Emerg Med J. 2019 Mar;36(3):171-5. DOI: 10.1136/emermed-2018-207872
[38] Bledsoe BE, Sweeney RJ, Berkeley RP, Cole KT, Forred WJ, Johnson LD. EMS provider compliance with infection control recommendations is suboptimal. Prehosp Emerg Care. 2014;18(2):290-4. DOI: 10.3109/10903127.2013.851311
[39] Chen YC, Sheng WH, Wang JT, Chang SC, Lin HC, Tien KL, Hsu LY, Tsai KS. Effectiveness and limitations of hand hygiene promotion on decreasing healthcare-associated infections. PLoS One. 2011;6(11):e27163. DOI: 10.1371/journal.pone.0027163
[40] Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, Perneger TV. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000 Oct;356(9238):1307-12. DOI: 10.1016/s0140-6736(00)02814-2
[41] Miramonti C, Rinkle JA, Iden S, Lincoln J, Huffman G, Riddell E, Kozak MA. The prevalence of methicillin-resistant staphylococcus aureus among out-of-hospital care providers and emergency medical technician students. Prehosp Emerg Care. 2013;17(1):73-7. DOI: 10.3109/10903127.2012.717169
[42] Elliott MJ, Kellum MT, Tenover FC, Pettriess RL. Nasal carriage of methicillin-susceptible and methicillin-resistant Staphylococcus aureus among paramedics in the Sedgwick County emergency medical service in Wichita, Kansas. Infect Control Hosp Epidemiol. 2002 Feb;23(2):60-1. DOI: 10.1086/503454
[43] Blue R, Whittle JS, Thundiyil JG, Silvestri S, Ralls G, Sirotkin L, Ladde J, Weber K, Giordano P. Prevalence of methicillin resistant Staphylococcus aureus in out-of-hospital health care providers. Ann Emerg Med. 2010;56:S87. DOI: 10.1016/j.annemergmed.2010.06.312
[44] Roberts MC, Soge OO, No D, Beck NK, Meschke JS. Isolation and characterization of methicillin-resistant Staphylococcus aureus from fire stations in two northwest fire districts. Am J Infect Control. 2011 Jun;39(5):382-9. DOI: 10.1016/j.ajic.2010.09.008




