Human skin equivalent as an alternative to animal testing
Heike Mertsching 1Michaela Weimer 1
Silke Kersen 1
Herwig Brunner 1
1 Fraunhofer Institute of Interfacial Engineering and Biotechnology, Department Cell Systems, Stuttgart, Germany
Abstract
The 3-D skin equivalent can be viewed as physiologically comparable to the natural skin and therefore is a suitable alternative for animal testing. This highly differentiated in vitro human skin equivalent is used to assess the efficacy and mode of action of novel agents. This model is generated from primary human keratinocytes on a collagen substrate containing human dermal fibroblasts. It is grown at the air-liquid interface which allows full epidermal stratification and epidermal-dermal interactions to occur.
Future emphasis is the establishment of different test systems to investigate wound healing, melanoma research and infection biology. Key features of this skin model are that it can be used as an alternative for in vivo studies, donor tissue can be tailored to the needs of the study and multiple analyses can be carried out at mRNA and protein level.
Driven by both ethical and economical incentives, this has already resulted in a shift of the test strategies used by the Pharmaceutical Industry in the early drug development process as reflected by the increased demand for application of cell based assays. It is also a suitable model for testing a wide variety of endpoints including cell viability, the release of proinflammatory mediators, permeation rate, proliferation and biochemical changes.
Keywords
alternative testing, skin equivalent, permeation
Introduction
Tissue engineering is an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function. It offers the potential to create replacement structures from biodegradable scaffolds and autologous cells for reconstructive surgery.
The living skin equivalent, a three-dimensional organotypic model, has been widely used to investigate many aspects of cutaneous biology. Reconstructed human skin equivalents are the models that most closely mimic normal human skin. They allow the topical application and skin irritancy testing of a great variety of products used in daily life. The major requirements for skin penetration screening are the presence of a competent skin barrier. In native skin the first barrier function is carried out by the outside layer of the skin, the stratum corneum. The second barrier is the basement membrane In studies with skin equivalent cultures, it has become evident that this two barrier function is affected. It is until now unclear whether nanoparticles will penetrate the skin.
Some manufacturers of consumer products, particularly cosmetics, and perhaps in the future foodstuffs, may utilise the advantages derived from including particulate materials in nano size in these products to give improved or additional functionality. These nanoparticles will be free rather than fixed, although their reactivity (and thus toxicity) may be influenced by coatings. Basically cosmetic products is an area where nanoparticles of oxides of zinc, titanium and iron are being used, and where there are concerns that they might penetrate through the protective layers of the skin and cause reactions with UV light that result in damage to DNA in skin cells. And these corrosion causes in higher irritation potential of cosmetic products or in early skin aging.
Methods
Design of the basic 3-D skin model
The development of a skin model requires firstly the isolation and growth of dermal fibroblasts and epidermal keratinocytes from human bioptic tissues. This highly differentiated in vitro human skin equivalent model is used to assess the efficacy and mode of action of novel agents. The skin equivalent is generated from primary human keratinocytes on a collagen gel substrate containing human dermal fibroblasts. It is grown at the air-liquid interface which allows full epidermal stratification and epidermal-dermal interactions to occur.
Figure 1 [Fig. 1] demonstrates the natural skin in comparison with the skin equivalent.
Figure 1: Histological cross section of human skin, and of the three dimensional skin equivalent with
stratum corneum
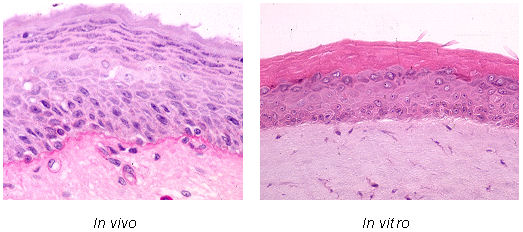
Immunohistochemical staining for cell characterization was performed by use of the avidin-biotin-peroxidase technique. Keratinocytes were characterized by the expression of Keratin 10, 14, 19, Filaggrin and Involucrin. Streptavidin-peroxidase conjugate was applied, and final staining was performed with diaminobenzidine.
Results and discussion
We applied our skin equivalent in many different areas such as:
1. Using the skin equivalent for in-vitro toxicity tests
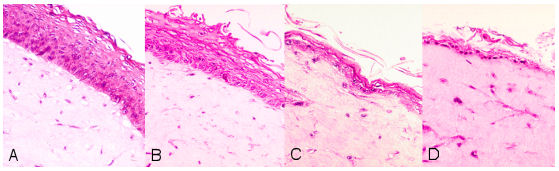
The irritation effect of different test substances was examined after topical application of the samples on the surface of the skin equivalent. A cell damage which can be attributed to the substance was photometrically quantified over the reduction from non toxic Tetrazolium salt to water-soluble Formazan (Figure 2 [Fig. 2]).
Figure 2: Histological cross section: control (A) after 20% SDS-application for 2 sec (B), 30 sec (C) and 90 sec (D)
2. Using the skin equivalent as an in-vitro tumor model
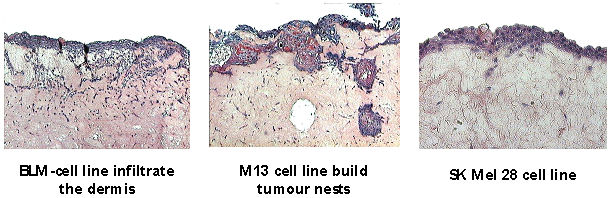
The invasion of malignant cells in normal tissues is a fundamental characteristic for progressing and the formation of metastases. In order to simulate the invasion in vitro, different tumor cell lines are co-cultivated. Therefore it is possible to analyze the same influencing variables such as growth factors on the invasion behavior of tumors and to test possible therapeutics (e.g. inhibitors) (Figure 3 [Fig. 3]).
Figure 3: Different aggressive tumor cells on the 3-D skin equivalent
3. Using the skin equivalent for testing in-vitro infection
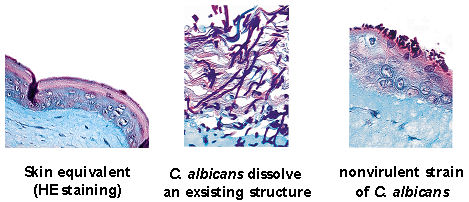
Infection of the reconstituted skin (A) with a clinical isolate of C. albicans (B) and an avirulent strain (C). The clinical strain penetrates the protective layer of keratinocytes and invades through the epithelial cell layers into the matrix, leading to disintegration of the model system after 48 h (B). The avirulent mutants do not form hyphae and show no ability to invade the tissue. Candida albicans was only detected on the tissue surface (C). The infection models can also be applied for drug screening [1] (Figure 4 [Fig. 4]).
Figure 4: Different strains of
Candida albicans
and their potential to penetrate the skin
4. Using the skin equivalent as an in-vitro wound healing model
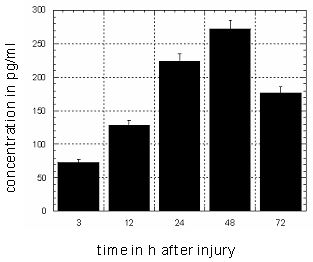
A wound could be initiated by mechanical effect of an Erb:YAG laser in the artificial skin. The defective region was activated by stimulated keratinocytes of the epidermis to refill the wound (Figure 5 [Fig. 5]). Parallel the IL-1α expression was measured during the wound healing in the medium by ELISA (Figure 6 [Fig. 6]). Uninjured skin equivalents served as controls.
Figure 5: Wound healing process after injury with a laser
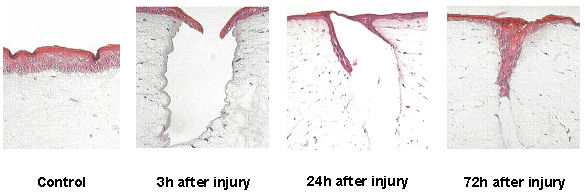
Figure 6: Analyzing of Interleukin 1α in the supernatant of the medium during wound healing process
By the physiological similarity with the natural skin the 3-D human skin equivalent is suitable as a test system for:
- determination of the irritation potential of different substances
- pharmacological analysis (e.g. wound healing)
- analysis of infection and invasion of different pathological microorganisms
- target screening
- immunological, histological and molecular-biological analysis
- proof of efficacy and quality control
- penetration- und permeation studies
- development of bio-chips for tumor diagnostics or other skin diseases
- development of medical devices, e.g. laser assisted diagnostic device for melanoma
Pharmaceutical research is hampered by limited predictive value of routinely applied in vitro and in vivo drug screening models for clinical efficacy. In drug development, the common approach of pharmaceutical industry is to screen small-molecule libraries for function and toxicity in biochemical based or ligand binding high throughput essays [2]. In general enzymes and 2-dimensional cell lines are used in those cell-based assays. The obtained results are of limited biological relevance, since the 2-dimensional cell systems do not adequately mimic the 3-dimensional environment in healthy and tumour tissues [3].
This model offers the possibility to simulate physiological drug application and a human 3-D test system to established nanomaterials/systems for cancer research/therapy.
References
[1] Dieterich C, Schandar M, Noll M, Johannes FJ, Brunner H, Graeve T, Rupp S. In vitro reconstructed human epithelia reveal contributions of Candida albicans EFG1 and CPH1 to adhesion and invasion. Microbiology. 2002:497-506.[2] Sundberg SA. High-throughput and ultra- high-throughput screening: solution and cell based approaches. Curr Opin Biotechnol. 2000;11:47-53.
[3] Balis FM. Evolution of anticancer drug discovery and the role of cell-based screening. J Nat Cancer Inst. 2002;94:78-9.




