Comparison of the antioxidant potential in urine, saliva and skin
Hicham Benkhai 1Franziska Köhler 1
Jürgen Lademann 2
Sandra Lemanski 3
Manfred Bornewasser 3
Elke Below 4
Harald Below 1
Axel Kramer 1
1 Institute of Hygiene and Environmental Medicine, University Medicine Greifswald, Germany
2 Charité-University Medicine Berlin, Department of Dermatology and Allergology, Berlin, Germany
3 Institute of Psychology, Ernst Moritz Arndt University, Greifswald, Germany
4 Institute of Forensic Science, Ernst Moritz Arndt University, Greifswald, Germany
Abstract
Aim: Free radicals, oxidative stress and their possible consequences for health are becoming increasingly important in modern medicine. Reactive species influence the organism, potentially causing oxidative cell damage. They can be produced by exogenous sources, or be a product of a variety of not only physiological metabolic processes, such as immune response, but also pathological processes. The antioxidant protection system protects the organism from oxidative damage caused by reactions producing an excess of free radicals. The analysis of antioxidant potential (AOP) is therefore becoming increasingly important for the diagnosis of individual vitality.
Method: The photochemoluminescence method was used to measure the AOP in urine and saliva, spectrometry was employed to measure the β-carotene content of the skin. In addition, it was investigated whether the AOPsaliva correlated with the AOPUurine (uric-acid independent AOP) as well as the β-carotene content of the skin.
Results: The AOP was significantly higher in urine than in saliva, and both values were significantly positively correlated with each other. However, there was no significant correlation to the β-carotene content of the skin.
Discussion: The components of the AOPUurine are accumulated over time (night), whereas AOP measurement in saliva is like a snapshot, which explains why AOPUurine was significantly higher than AOPsaliva, although the two parameters are correlated with each other. β-carotene is a fat-soluble antioxidant, whereas in our study, only water-soluble antioxidants were determined in the urine. This explains why there is no positive correlation between β-carotene of the skin and AOP.
Conclusion: For the characterization of the AOP in epidemiological studies, we recommend determining the AOPUurine and parallel to this, the β-carotene content of the skin.
Keywords
antioxidant potential, free radicals, oxidative stress, beta-carotene, reactive oxygen species, urine, saliva, photochemoluminescence, antioxidants
Introduction
Radical generation can be triggered exogenously (e.g., UV and ionizing radiation) and endogenously (e.g., mitochondrial respiratory chain and immune system) [1], [2]. An excess of free radicals due to increased formation or inadequate antioxidant mechanisms is harmful to the organism [3]. Because of their unpaired electrons and the associated instability and reactivity, radicals can cause damage in biological systems with the consequence of changing their structure and function. The undesirable effects include the inactivation of NO by direct chemical reaction with reactive oxygen species (ROS), and oxidative damage to cellular components such as DNA and proteins [4]. These effects are possibly related to the development of cardiovascular diseases, neurodegenerative diseases, cancer, and aging processes [5], [6], [7].
The antioxidant system is responsible for minimizing oxidative cell damage in the body caused by free radicals. Ideally, it creates a balance which permits the beneficial effects of free radicals while preventing the harmful ones. The system includes enzymatic antioxidants (e.g., superoxide dismutase) and non-enzymatic exogenous and endogenous antioxidants, which can be hydrophilic or lipophilic (e.g., ascorbic acid and glutathione, resp.) [8].
AOP in urine and saliva can be determined by different methods, i.e., photochemoluminescence (PCL) [6], [7]. Another method to determine the antioxidant status is the measurement of the β-carotene content of the skin [9]. The antioxidant balance of an individual is influenced by diet, sports, stress, among others [10], [11], [12]. Up to now, the AOP in saliva, urine and skin were not determined in parallel in the same person. Therefore, the focus of the study was to compare the AOP of the 3 sampled media.
Methods
Nine psychology students (7 women, 2 men, 20 to 25 years old) participated in the three-day study. Every morning, fasting samples of spot urine and saliva from each participant were collected to determine AOPsaliva and AOPUurine. The samples were coded and then immediately frozen at –80°C. While in saliva only the AOP was analyzed, in the urine samples, both AOPU and the creatinine content were determined, where the latter was adjusted for volume excreted. Before analysis, the samples were allowed to thaw overnight in the refrigerator and were centrifuged after mixing briefly (5000 rpm) in order to separate suspended matter. The centrifuged urine was diluted 1:10 and incubated for 5 min with uricase to eliminate uric acid. Subsequently, the AOP was measured by using Photochem® and the ACW kit (Analytik Jena, Germany) [12], [13]. The chemicals had pro analysis quality. For all investigations, high-purity water was used (Reinstwasser-System, SG Wasseraufbereitung und Regenerierstation GmbH, Barsbüttel, Germany). For creatinine, we used the creatinine-DRI® Test Detect (Micro Genetics GmbH, Passau, Germany). The AOP was calculated using the following formula:
AOP (mg/g creatinine) = AOP (mg/dl) x 1000/creatinine (mg/dl)
Parallel to the daily sampling, we determined the β-carotene content (scale 0–10) of the skin by using biozoom® (Opsolution Nanophotonics GmbH, Kassel, Germany) [14]. The participants were instructed not to apply any skin cream in each morning before measurement.
Statistical analysis was performed with the program PASW® Statistics 18 (IBM). The parameters were intended for descriptive statistics, and Pearson’s correlation was calculated and tested for significance.
Results
The AOPUurine was significantly higher than in saliva. The mean value of β-carotene, 4.0, was slightly lower than expected (Table 1 [Tab. 1]).
Table 1: Descriptive statistics of the study (n=9)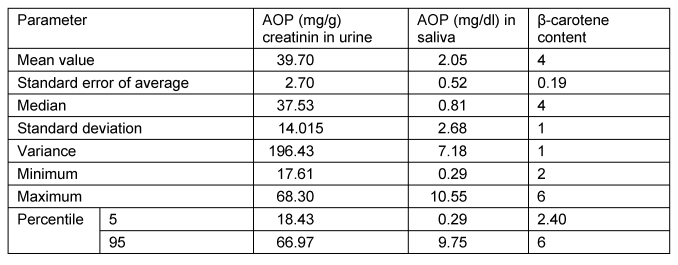
The data analysis shows a significant positive correlation between the parameters AOPUurine and AOPsaliva at p<0.05 (Table 2 [Tab. 2]). In contrast, the values of β-caroteneskin with those of AOPUurine show a weak positive correlation, but this was not significant. The AOPsaliva shows a slight negative correlation with the values of skin measurement, but the difference was not significant (Table 2 [Tab. 2]).
Table 2: Correlation analysis of the parameters AOPsaliva, AOPUurine and β-carotene (skin)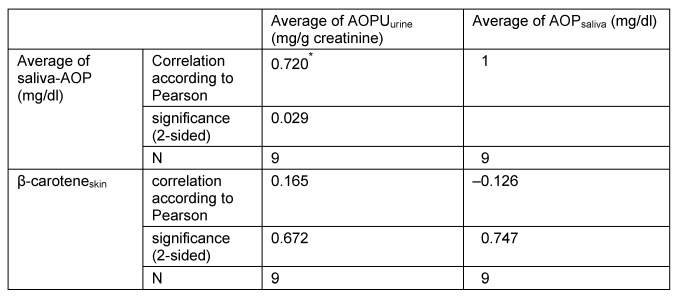
Discussion
Antioxidant capacity was determined in materials which are non-invasively accessible, that is, in saliva, urine and skin. The intention was to use conditions suitable for future epidemiological studies. The ideal would be to collect 24-h urine. Since this was not logistically realizable, spontaneous urine was used and normalized by creatinine content in urine; this method has been proven in studies on iodine deficiency screening [15]. Influences of age, sex, lifestyle and nutrition were not considered in the pilot study, because the purpose was to compare the measured parameters. While urine is an excretion with accumulation of the excreted substances over time, saliva is a secretion, in which components can be exogenously influenced and endogenously metabolized, thus the composition can change quickly. This may explain the finding that the AOPUurine is at least 10 times higher than AOPsaliva. However, these two parameters were correlated significantly with each other. This result supports the hypothesis that urine and saliva are suitable for determining the AOP. Since saliva production is difficult to standardize and more susceptible to external influences than urine, the standard deviation in saliva is about 5.2 higher than in urine.
β-carotene is a fat-soluble antioxidant. In the urine, only water-soluble antioxidants were analyzed. This may be the reason why there is no positive correlation between β-carotene of the skin and the AOP of saliva and urine.
Conclusions
Our results suggest that in epidemiological studies, the AOPUurine and, in parallel, the β-carotene content of the skin are suitable for characterizing the antioxidative status, whereas the AOPsaliva is dispensible.
Notes
Competing interests
The authors declare that they have no competing interests.
Acknowledgment
This work was supported by the project Flex4Work, sponsored by BMBF and ESF (FKZ 01FH09127). We thank the company Opsolution Nanophotonics GmbH for providing the equipment biozoom©.
References
[1] Lademann J, Patzelt A, Schanzer S, Richter H, Meinke MC, Sterry W, Zastrow L, Doucet O, Vergou T, Darvin ME. Uptake of antioxidants by natural nutrition and supplementation: pros and cons from the dermatological point of view. Skin Pharmacol Physiol. 2011;24(5):269-73. DOI: 10.1159/000328725[2] Jeon HY, Kim JK, Seo DB, Cho SY, Lee SJ. Beneficial effect of dietary epigallocatechin-3-gallate on skin via enhancement of antioxidant capacity in both blood and skin. Skin Pharmacol Physiol. 2010;23(6):283-9. DOI: 10.1159/000313542
[3] Lademann J, Schanzer S, Meinke M, Sterry W, Darvin ME. Interaction between carotenoids and free radicals in human skin. Skin Pharmacol Physiol. 2011;24(5):238-44. DOI: 10.1159/000326074
[4] Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245-313. DOI: 10.1152/physrev.00044.2005
[5] Dix TA, Aikens J. Mechanisms and biological relevance of lipid peroxidation initiation. Chem Res Toxicol. 1993;6(1):2-18. DOI: 10.1021/tx00031a001
[6] Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90(17):7915-22.
[7] Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344(8924):721-4. DOI: 10.1016/S0140-6736(94)92211-X
[8] Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215(2):213-9. DOI: 10.1111/j.1432-1033.1993.tb18025.x
[9] Lademann J, Schanzer S, Meinke M, Sterry W, Darvin ME. Interaction between carotenoids and free radicals in human skin. Skin Pharmacol Physiol. 2011;24(5):238-44. Doi: 10.1159/000326074.
[10] Popov I, Lewin G. Photochemiluminescent detection of antiradical activity. VI. Antioxidant characteristics of human blood plasma, low density lipoprotein, serum albumin and amino acids during in vitro oxidation. Luminescence. 1999;14(3):169-74. DOI: 10.1002/(SICI)1522-7243(199905/06)14:3<169::AID-BIO539>3.0.CO;2-K
[11] Popov IN, Lewin G. Photochemiluminescent detection of antiradical activity: II. Testing of nonenzymic water-soluble antioxidants. Free Radic Biol Med. 1994;17(3):267-71. DOI: 10.1016/0891-5849(94)90082-5
[12] Benkhai H. Untersuchungen zur Wertigkeit des Summenparameters Antioxidatives Potential für medizinische Fragestellungen einschließlich Stressbelastung. Greifswald: Ernst-Moritz-Arndt-Universität Greifswald, Institut für Hygiene und Umweltmedizin; 2010.
[13] Analytik Jena AG. Bestimmung der wasserlöslichen antioxidativen Kapazität in Blutplasma (ACW). 2000.
[14] Darvin ME, Sandhagen C, Koecher W, Sterry W, Lademann J, Meinke MC. Comparison of two methods for non-invasive determination of carotenoids in human and animal skin: Raman spectroscopy versus reflection spectroscopy. J Biophoton. accepted.
[15] Zöllner H, Als C, Gerber H, Hampel R, Kirsch G, Kramer A. Screening for iodine deficiency: Iodide concentration or creatinine quotient in random urine? GIT Laboratory Journal. 2001;5(3):138-9.




