Minimum inhibitory (MIC) and minimum microbicidal concentration (MMC) of polihexanide and triclosan against antibiotic sensitive and resistant Staphylococcus aureus and Escherichia coli strains
Ojan Assadian 1Katrin Wehse 2
Nils-Olaf Hübner 2
Torsten Koburger 3
Simone Bagel 4
Frank Jethon 5
Axel Kramer 2
1 Clinical Institute for Hospital Hygiene, Medical University of Vienna, Vienna, Austria
2 Institute of Hygiene and Environmental Medicine, University Medicine Greifswald, Germany
3 Hygiene-North GMBH, Greifswald, Germany
4 Antiinfectives Intelligence, Clinical Microbiological Research and Communication GmbH, Campus Fachhochschule Bonn-Rhein-Sieg, Bonn, Germany
5 Fresenius Kabi Deutschland GmbH, Bad Homburg, Germany
Abstract
Background: An in-vitro study was conducted investigating the antimicrobial efficacy of polihexanide and triclosan against clinical isolates and reference laboratory strains of Staphylococcus aureus and Escherichia coli.
Methods: The minimal inhibitory concentration (MIC) and the minimal microbicidal concentration (MMC) were determined following DIN 58940-81 using a micro-dilution assay and a quantitative suspension test following EN 1040. Polihexanide was tested in polyethylene glycol 4000, triclosan in aqueous solutions.
Results: Against all tested strains the MIC of polihexanide ranged between 1–2 µg/mL. For triclosan the MICs varied depending on strains ranging between 0.5 µg/mL for the reference strains and 64 µg/mL for two clinical isolates. A logRF >5 without and logRF >3 with 0.2% albumin burden was achieved at 0.6 µg/mL triclosan. One exception was S. aureus strain H-5-24, where a triclosan concentration of 0.6 µg/mL required 1 minute without and 10 minutes with albumin burden to achieve the same logRFs. Polihexanide achieved a logRF >5 without and logRF >3 with albumin burden at a concentration of 0.6 µg/mL within 30 sec. The exception was the North-German epidemic MRSA strain, were an application time of 5 minutes was required.
Conclusion: The clinical isolates of E. coli generally showed higher MICs against triclosan, both in the micro-dilution assay as well in the quantitative suspension test than comparable reference laboratory strains. For polihexanide and triclosan strain dependant susceptibility was shown. However, both antimicrobial compounds are effective when used in concentrations common in practice.
Keywords
polihexanide, triclosan, antimicrobial, inhibitory concentration, microbicidal concentration
Introduction
Due to their different chemical structure, their different use in clinical practice and their different antimicrobial capacity the matter of antibiotic resistance does not apply to antiseptics at the moment. So far, for microbicidal antiseptics no clinically relevant resistance is documented [1], [2], [3]. For microbistatic antiseptics, however, the potential risk for development of resistance can not be excluded. Examples for microbistatic antiseptics include benzalkonium chloride [4], Cetylpyridinium chloride [5], chlorhexidine and triclosan [6], [7]. For hexachlorophene [1] and chlorhexidine [8] a plasmid-coded resistance was shown already.
Furthermore, for some antiseptics and antibiotics, increased resistance against antiseptics was correlated with increased antibiotic resistance [9], [10]; however, increased antibiotic resistance has not been associated with antiseptic resistance so far. Russel et al. [11] could demonstrate in an in-vitro study that after Pseudomonas stutzeri was exposed to increasing concentrations of chlorhexidine after multiple passages, a stable chlorhexidine resistance was achieved. Simultaneously resistance against benzalkonium chloride, cetylpyridinium chloride and triclosan and a number of antibiotics was increased as well. For Mycobacterium smegmatis our study group could show a parallel increase in resistance for isoniazid and triclosan [7].
For the majority of antiseptic compounds the first step for their antimicrobial action is penetration into the bacterial cell. If this penetration is minimized or inhibited, resistance will result. Bacteria are able to alter their cell’s permeability by changing its lipid content, the composition of the outer membrane proteins, plasmid-coded mucous production or by altering the affinity of their efflux pumps. If the efflux pump has an unspecific affinity to several compounds, resistances will result. Example for an efflux pump with a broad substrate spectrum is AcrAB of Escherichia coli which is also responsible for the efflux activity of the membrane-pore-protein TolC, able of transporting tetracycline, chloramphenicol, fluoroquinolones, β-lactams, novobiocin, erythromycin, ethidium bromide, crystal violet and Acriflavin [12], [13].
In contrast to antibiotics where antibiogramms are generated in course of routine clinical microbiological diagnostics for a broad range of antibiotics and bacteria, such routine data is not available for antiseptics and knowledge on antimicrobial efficacy is based only on selected, most often historic, literature. In light of the possibilities for development of resistance against antiseptics it deems useful to control the antimicrobial activity of frequently used antiseptics and possible changes in regular intervals.
Therefore we conducted an in-vitro study investigating the current antimicrobial efficacy of the frequently used skin, mucous membrane and wound antiseptics polihexanide and triclosan against clinical isolates and defined laboratory strains of Staphylococcus aureus and E. coli.
Methods
Tested antimicrobial compounds
For polihexanide, we used the commercially available product Lavasept® (Fresenius AG, Bad Homburg, Germany) which contains 200 µg/mL polihexanide and 10 µg/mL macrogolum 4000. Because triclosan as pure substance (Irgasan®DP 300; CAS No 88032-08-0; Ciba AG, Basel, Switzerland) is not solvable in water, we diluted the product in dimethylsulfoxid (DMSO) and 9.2% ethanol according to the manufacturer’s recommendation. In a separate pilot experiment, we could rule out that 9.2% ethanol itself possesses antimicrobial properties.
Tested clinical isolates and laboratory strains
The following bacteria were tested using the micro-dilution test: E. coli ATCC 25922; E. coli AG 100 wild type (K-12 argE3 thi-1 rpsL xyl mtl Δ(gal-uvrB) supE44) [14], [15]; E. coli AGT11, containing fabI mutation with resistance against triclosan; exchange of amino acid in the enoyl-reductase in position 93 [16]; E. coli AGT 11K (AcrAB::kan, deletion of the E. coli efflux pump AcrAB) [14]; E. coli AGT 11 Kan (AmarCORAB::kan, constructed by replacement of a chromosomal 1.24-kb BspHI fragment of the mar locus in AG100 by homologous recombination with the kanamycin resistance cassette (Kanr) from pKMN33; deletion of the regulator gene which activate the efflux pump) [17]; S. aureus ATCC 29213, MRSA strains H-5-18, H-5-19, H-5-20, H-5-24, H-5-26, H-5-27, and H-5-31 (University Clinic Bonn, Germany), MRSA epidemic strain North-Germany, Niedersachsen and Berlin (Robert Koch Institute, Wernigerode, Germany); VISA strains Ve 13985, Ve 1177/98, BK 13230, BK 1704/98, 18 A 026, and 20 A 063 (Robert Koch Institute, Wernigerode, Germany).
Additionally to the micro-dilution test, S. aureus ATCC 29213, MRSA H-5-24, MRSA epidemic strain North-Germany, E. coli ATCC 25922 and E. coli AGT 11 were tested in a quantitative suspension test.
Culture media and neutralizing agents
As polihexanide precipitates on Mueller-Hinton agar, micro-organisms were cultured on iso-sensitest bouillon (Oxoid, Darmstadt, Germany), tryptone soya agar and broth, respectively (Oxoid, Wesel, Germany). As neutralizer for polihexanide 3% (w/v) Tween 80 (Serva, Heidelberg, Germany), 3% (w/v) saponine (Fluka, Buchs, Switzerland), 0.1 % (w/v) histidine (Serva, Heidelberg, Germany), and 0.1% (w/v) cysteine (Merck, Darmstadt, Germany) was used. As neutralization of triclosan was not possible with the usual neutralization solution, we used egg yolk (sterile egg yolk diluted to 50% by sterile distilled water) instead. However, only triclosan concentrations of 0.6 µg triclosan/L and less were possible. Therefore, this concentration also was the maximum tested concentration in the quantitative suspension test.
Determination of the Minimum Inhibitory Concentration (MIC)
The MIC was determined following DIN 58940-81 [18] using a micro-dilution assay. Briefly, using colonies from a fresh overnight culture an inoculum of 1x105 cfu/mL in the final medium was prepared. 50 µl of the inoculum were added to 50 µl of the respective antimicrobial test-dilution and incubated at 36°C ± 1°C for 20h ± 2h.
Determination of the Minimum Microbicidal Concentration (MMC)
The MMC was determined using the quantitative suspension test following Pitten et al. [19] with and without bio-burden. For each assay, 0.1 ml of the test organism (108–109 cfu/mL) in tryptone soya broth was transferred from a fresh overnight culture into a test tube, mixed with 1 ml distilled sterile water (test without bio-burden) or, in parallel series, with 1 ml of 0.2% bovine serum albumin (test without bio-burden), and transferred in 9 ml of the test solution. At the final time of action (30 sec, 60 sec, 5 min, 10 min, 60 min, respectively) 1 ml of the test mixture was transferred into 9 ml tryptical-soy-broth with addition of the respective neutralizer. After 5 minutes of neutralisation, serial dilutions were plated on trypticase-soy-agar. Colonies were counted after 48h. The log10 reduction factor (RF) for each application time was calculated using the formula: log10 (control) – log10 (test sample). All experiments were performed in triplicate. Both antiseptics were tested at concentrations of 2 µg/mL, 0.2 µg/mL and 0.02 µg/mL.
Statistical analysis
All assays were repeated 6-fold, and number of organisms were averaged as mean cfu/ml and expressed as log10. The log10 reduction factor (logRF) was calculated as log10 of the pre-value minus log10 of the post-value.
Results
Microbistatic activity
For S. aureus no difference was observed in the antimicrobial activity of polihexanide at concentrations of 1 and 2 µg/mL against ATCC reference strains, 10 MRSA isolates and 6 VISA strains (Table 1 [Tab. 1]). Triclosan could be tested only against the ATCC strains and the epidemic North-German MRSA strain. Compared to MRSA strains the antimicrobial activity of triclosan was 512 times lower.
Table 1: MIC (µg/mL) of polihexanide and triclosan against S. aureus and different MRSA and VISA-strains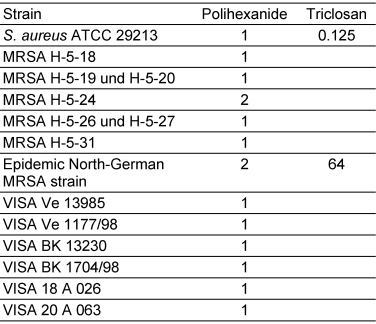
All E. coli ATCC reference strains and the E. coli wild type AG 100 were inhibited by triclosan concentrations ranging between 0.5 and 1 µg/mL. E. coli AGT 11 with a mutation in its enoylacyl-carrierprotein-reductase and E. coli AGT Kan11, a strain identical to E. coli AGT 11 but with additionally defect efflux pump regulation showed an increased MIC by the factor 128 against triclosan. For E. coli AGT 11K, again a strain identical to E. coli AGT 11 but additionally with switched-off efflux pump AcrAB triclosan resistance was decreased by 32 times (Table 2 [Tab. 2]). The minimum microbistatic concentration for polihexanide was 2 µg/mL against all tested E. coli strains.
Table 2: MIC (µg/mL) of polihexanide and triclosan against E. coli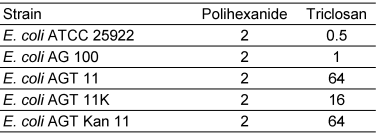
Microbicidal activity
In the quantitative suspension test polihexanide showed superior activity than triclosan (Table 3 [Tab. 3]). S. aureus ATCC strains did not differ in their susceptibility against triclosan as compared to the North-German epidemic MRSA strain. The minimum requirements of a logRF >5 without and logRF >3 with 0.2% albumin burden within 5 minutes application time [19] were achieved at a concentration of 0.6 µg/mL triclosan. The exception was MRSA strain H-5-24, where a triclosan concentration of 0.6 µg/mL was able only after 60 sec without and 10 minutes with albumin burden to achieve a logRF >5 or a logRF >3 reduction, respectively. The difference between E. coli ATCC 25922 und E. coli AGT 11 was only minute, for both strains a concentration of 0.6 µg/mL triclosan at an application time of 60 sec was needed to achieve a logRF >5 without and a logRF >3 with albumin-burden.
Table 3: Time and concentration dependant bactericidal activity of polihexanide and triclosan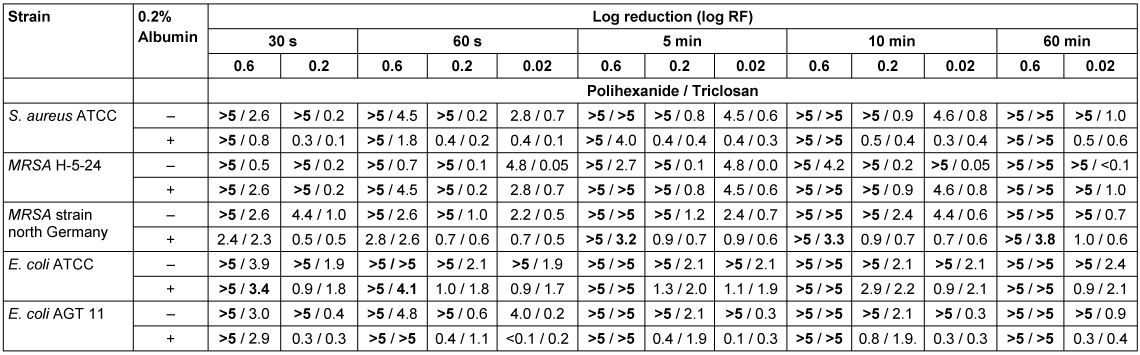
Polihexanide achieved a logRF >5 without and lofRF >3 with albumin burden at concentrations of 0.6 µg/mL within 30 sec, respectively, for S. aureus. The only exception was the North-German epidemic MRSA strain, were an application time of 5 minutes was required. MRSA H-5-24 did not differ in its susceptibility from the ATCC reference strains. E. coli AGT 11 was only lesser susceptible to polihexanide as compared to E. coli ATCC 25922 under the presence of an albumin burden. Without albumin burden a logRF >5 was achieved with a concentration of 0.2 µg/mL and an application time of 30 sec, or 0.02 µg/mL and 60 sec, respectively. With albumin burden even an application time of 1 minute was not sufficient for a concentration of 0.02 µg/mL polihexanide to achieve a logRF >3.
Discussion
In order to assess the efficacy of the tested antimicrobial compounds polihexanide and triclosan, our results must take their usual clinically used concentrations into account. Triclosan usually is used in liquid soap at concentration of 2 to 5 µg/mL, in toothpaste starting from 3 µg/mL and in hand disinfectants ranging from 2 to 20 µg/mL [7].
Wound antiseptics containing polihexanide as their active ingredient contain concentrations of 0.2 to 1 µg/mL and sanitizers 1 to 2 µg/mL. Our results showed that the required minimum concentration for triclosan and polihexanide are 0.6 µg/mL, and therefore, concentrations used in clinical still are far above the MIC demonstrated for various clinical isolates and laboratory reference strains. The focus of our study, however, was not to assess the antimicrobial efficacy in clinical practice, but rather to explore differences in susceptibility within various antibiotic susceptible strains of S. aureus and E. coli in order to detect possible induction of co-resistance against antiseptics.
The results of our study showed that the susceptibility of the epidemic North-German MRSA strain against triclosan was reduced as compared to antibiotic susceptible strains. Also MRSA H-5-24 showed reduced susceptibility against triclosan. As expected, triclosan was highly effective against the E. coli ATCC strain. In contrast, clinical E. coli isolates showed decreased susceptibility against triclosan. The mode of action of triclosan is based on the inhibition of the biosynthesis of lipid acid and enoylacyl-carrierprotein-reductase [7]. For E. coli to become resistant against triclosan, a single change of 1 amino acid in the reductase enzyme is sufficient resulting in a 32- to 128-fold MIC increase.
Another reason for the increased resistance of E. coli against triclosan could also be an increased activity of the efflux pump AcrAB-TolC located in the bacterial cell membrane. E. coli mutants with increased efflux pump activity showed higher MIC values against various antibiotics as well as triclosan than strains with normal efflux pump activity. This correlation was also observed earlier by McMurry et al. [14] and was explained by the fact that affected compounds are substrates of the same membrane pump AcrAB-TolC. Our observation of a multiple antibiotic-resistance-phenotype combined with an increase in triclosan MIC by 1 to 2 titres in some clinical E. coli isolates confirms this possibility.
With one exception MICs of antibiotic resistant and susceptible strains against polihexanide did not differ showing that polihexanide has a different mode of action against bacteria [20]. Furthermore, the observed low MICs for polihexanide allow the conclusion that polihexanide is not an substrate for above efflux pumps. Based on the presented in-vitro results and clinical observations [21], it is explainable why polihexanide is a very effective wound antiseptic. Our data show that polihexanide easily is able to kill even antibiotic resistant bacteria commonly found as the cause of wound infection. Currently, it can be concluded that unlike triclosan, polihexanide is not transported outside of the bacterial cell wall by the activity of efflux pumps, and therefore is not subject to development of possible bacterial resistance. Moreover, polihexanide is integrated into the bacterial cell membrane, which it irreversibly destroys.
However, as with all antimicrobial compounds, it seems prudent to limit its use to clinically justifiable indications.
Conclusion
The clinical isolates of E. coli generally showed higher MICs against triclosan, both in the micro-dilution assay as well in the quantitative suspension suspension test than comparable ATCC laboratory reference strains. For polihexanide and triclosan strain dependant susceptibility was shown. However, both antimicrobial compounds are effective when used in concentrations common in practice.
List of abbreviations
ATCC – American Type Culture Collection
logRF – log 10 Reduction factor
MIC – Minimum inhibitory concentration
MMC – Minimum microbicidal concentration
MRSA – Methicillin Resistant Staphylococcus aureus
VISA – Vancomycin intermediate sensitive Staphylococcus aureus
Notes
Authors’ contribution
AK and PR had the idea for the study, AK and PR planned and supervised the experiments, as well drafted the manuscript and analyzed and interpreted the data. KW, TK, and SB participated in the technical design of the study and performed experiments. All authors analyzed and interpreted the data. NOH, OA SB, and FJ participated in the study’s design and coordination, helped to draft the manuscript, and analyzed and interpreted the data. All authors have been involved in drafting the manuscript or revising it critically for important intellectual content and have read and approved the final manuscript.
Funding
The study was sponsored by Fresenius Kabi GmbH, Bad Homburg, Germany. The sponsors had no participation in the study design or interpretation of the results.
Conflicts of interest
Dr. Frank Jethon is a paid employee of Fresenius Kabi GmbH, Bad Homburg, Germany. Prof. Dr. Assadian, Dr. Bagel, Dr. Huebner, Prof. Dr. Kramer, Dr. Rudolph and Mrs. Wehse have no financial or other conflict of interest to declare.
References
[1] Lebek G. Genetische Grundlagen der Resistenzentwicklung von Mikroorganismen gegenüber Antiseptika bzw. Desinfektionsmitteln. In: Krasilnikow AP, Kramer A, Gröschel D, Weuffen W, eds. Handbuch der Antiseptik, Vol. I/2. Stuttgart: Fischer Verlag; 1981. p. 170-186.[2] Kramer A, Kedzia W, Lebek G, Grün L, Weuffen W, Poczta A. In-vitro- und In-vivo-Befunde zur Resistenzsteigerung bei Bakterien gegen Antiseptika und Desinfektionsmittel. In: Krasilnikow AP, Kramer A, Gröschel D, Weuffen W, eds. Handbuch der Antiseptik, Vol. I/4. Stuttgart: Fischer Verlag; 1984. p. 79-121.
[3] Kramer A, Roth B. Polihexanid. In: Kramer A, Assadian O, eds. Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung. Stuttgart: Georg Thieme Verlag; 2008. p. 788-793.
[4] Akimitsu N, Hamamoto H, Inoue R, Shoji M, Akamine A, Takemori K, Hamasaki N, Sekimizu K. Increase in resistance of methicillin-resistant Staphylococcus aureus to beta-lactams caused by mutations conferring resistance to benzalkonium chloride, a disinfectant widely used in hospitals. Antimicrob Agents Chemother. 1999;43(12):3042-3.
[5] Irizarry L, Merlin T, Rupp J, Griffith J. Reduced susceptibility of methicillin-resistant Staphylococcus aureus to cetylpyridinium chloride and chlorhexidine. Chemotherapy. 1996;42(4):248-52. DOI: 10.1159/000239451
[6] Kramer A, Assadian O, Müller G, et al. Octenidine-dihydrochloride, Chlorhexidine, Iodine and Iodophores. Vorabauszug aus Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung. 1. Aufl. Stuttgart: Georg Thieme Verlag; 2008.
[7] Kramer A, Schauer F, Assadian O, Heldt P. Triclosan. In: Kramer A, Assadian O, eds. Wallhäußers Praxis der Sterilisation, Desinfektion, Antiseptik und Konservierung. Stuttgart: Georg Thieme Verlag; 2008. p. 760-768.
[8] Kaulfers PM, Laufs R. Ubertragbare Formaldehydresistenz bei Serratia marcescens [Transmissible formaldehyde resistance in Serratia marcescens]. Zentralbl Bakteriol Mikrobiol Hyg B. 1985;181(3-5):309-19.
[9] Kampf G, Jarosch R, Rüden H. Limited effectiveness of chlorhexidine based hand disinfectants against methicillin-resistant Staphylococcus aureus (MRSA). J Hosp Infect. 1998;38(4):297-303. DOI: 10.1016/S0195-6701(98)90078-0
[10] Cottell A, Denver SP, Hanlon GW, Ochs D, Maillard JY. Triclosan-tolerant bacteria: changes in susceptibility to antibiotics. J Hosp Infect. 2009;72(1):71-6. DOI: 10.1016/j.jhin.2009.01.014
[11] Russel AD, Maillard JY, Furr JR. Possible link between bacterial resistance and use of antibiotics and biocides. PDA J Pharm Sci Technol. 1997;51(5):174-5. Available from: http://aac.asm.org/content/42/8/2151.long
[12] Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175(1):6299-313.
[13] Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178(20):5853-9.
[14] McMurry LM, Oethinger M, Levy SB. Triclosan targets lipid synthesis. Nature. 1998;394:531-2. DOI: 10.1038/28970
[15] George AM, Levy SB. Gene in the major cotransduction gap of the Escherichia coli K-12 linkage map required for the expression of chromosomal resistance to tetracycline and other antibiotics. J Bacteriol. 1983;155(2):541-8.
[16] McMurry LM, Oethinger M, Levy SB. Overexpression of marA, soxS, or acrAB produces resistance to triclosan in laboratory and in clinical strains of Escherichia coli. FEMS Microbiol Lett. 1998;166(2):305-9. DOI: 10.1111/j.1574-6968.1998.tb13905.x
[17] Maneewannakul K, Levy SB. Identification for mar mutants among quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40(7):1695-8.
[18] Empfindlichkeitsprüfung von mikrobiellen Krankheitserregern gegen Chemotherapeutika Teil 8: Mikrodilution; Allgemeine methodenspezifische Anforderungen (DIN 58940-8). Berlin: Beuth; 1998.
[19] Pitten FA, Werner HP, Kramer A. A standardized test to assess the impact of different organic challenges on the antimicrobial activity of antiseptics. J Hosp Infect. 2003;55(2):108-15. DOI: 10.1016/S0195-6701(03)00260-3
[20] Chawner JA, Gilbert P. Interaction of the bisbiguanides chlorhexidine and alexidine with phospholipid vesicles: evidence for separate modes of action. J Appl Bacteriol. 1989;66(3):253-8. DOI: 10.1111/j.1365-2672.1989.tb02476.x
[21] Roth B, Assadian O, Wurmitzer F, Kramer A. Wundinfektionen nach antiseptischer Primärversorgung kontaminierter traumatischer Wunden mit Polihexanid, PVP-Iod bzw. Wasserstoffperoxid. GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc58. Available from: http://www.egms.de/en/journals/dgkh/2007-2/dgkh000091.shtml




