Two-stage revision of implant-associated infections after total hip and knee arthroplasty
Martin Ellenrieder 1Robert Lenz 1
Maximilian Haenle 1
Rainer Bader 1
Wolfram Mittelmeier 1
1 Department of Orthopedics, University Hospital Rostock, Germany
Abstract
Septic loosening of total hip and knee endoprostheses gains an increasing proportion of revision arthroplasties. Operative revisions of infected endoprostheses are mentally and physically wearing for the patient, challenging for the surgeon and a significant economic burden for healthcare systems. In cases of early infection within the first three weeks after implantation a one-stage revision with leaving the implant in place is widely accepted. The recommendations for the management of late infections vary by far. One-stage revisions as well as two-stage or multiple revision schedules have been reported to be successful in over 90% of all cases for certain patient collectives. But implant associated infection still remains a severe complication. Moreover, the management of late endoprosthetic infection requires specific logistics, sufficient and standardized treatment protocol, qualified manpower as well as an efficient quality management. With regard to the literature and experience of specialized orthopaedic surgeons from several university and regional hospitals we modified a commonly used treatment protocol for two-stage revision of infected total hip and knee endoprostheses. In addition to the achievement of maximum survival rate of the revision implants an optimisation of the functional outcome of the affected artificial joint is aimed for.
Keywords
total hip and knee arthroplasty, implant-associated infection, treatment protocol, spacer
Introduction
Approximately 160,000 primary hip and 146,000 primary total knee arthroplasties are performed annually in Germany [1], [2]. Periprosthetic infections are described to occur in only 1% of all primary hip arthroplasties [3]. But in patients with existing risk factors such as diabetes, rheumatoid arthritis or after aseptic revision the risk of a late infection escalates up to >5% [4], [5], [6]. Due to the demographic changes implant-associated infections will gain increasing importance for patients, orthopaedic surgeons and healthcare systems. As a result, deep infection has taken over the second place of reasons for reoperation (25.9% of all reoperations) within the first two years after implantation [7]. The mean annual economic revision burden of total hip and knee arthroplasties has reached up to 20% [8]. Complicated septic revisions caused costs of 50,000 Euro or even more [9]. Hence, the clinical and economic impact of implant-associated infections after total hip and knee arthroplasty must be expected to gain significance in the future.
The period for grading an implant-related infection as “early infection” ranges from 3 weeks to 3 month postoperatively in literature [10], [11], [12]. Over the last few years there is a clear tendency to limit “early infections” to the first 3 to 4 weeks postoperatively because of a more profound knowledge of the pathophysiology of infection and the responsible microorganisms. Whether an early or late periprosthetic infection does occur this has crucial impact on the therapeutic approach. There is broad consensus to treat an early deep infection surgically (debridement, irrigation) but with retention of the femoral and acetabular component [6], [10], [12], [13]. However, an exchange of the femoral head and polyethylene insert should be performed since bacterial biofilms are found on these implants [14].
As opposed to this, the question how “late infections” should be treated is still undecided and lively discussed in the literature [3], [10], [12], [15], [16], [17], [18]. But there is a consensus that basically a treatment algorithm should be established for medical, economical and forensic reasons [16]. Besides the fact that the treatment needs to be standardized, this must hold true for the diagnostics of periprosthetic infections. Especially in cases of low-grade infections, infections with biofilm-producing bacteria or small colony variants and preceding antibiotic therapy, the classical diagnostic procedures (e.g. preoperative joint fluid aspiration, intraoperative swabs) are insufficient [10], [19], [20].
With regard to the literature and the experience of specialized orthopaedic surgeons from several university and regional hospitals we modified a commonly used treatment protocol [12]. A common concept allows to merge outcome data and to share expensive resources (e.g. laboratories, staff training, materials). Moreover, the presented algorithm is the basis for a prospective follow-up study which is especially focussed on infections with multi-resistant bacteria and treatment with improved temporary spacers.
Diagnostic and Treatment Protocol
A periprosthetic infection is assured if clinical signs such as a fistula or purulent wound secretion occur. Otherwise we combine the information from the clinical examination, laboratory (white blood cell count (WBC), C-reactive protein (CrP) and erythrocyte sedimentation rate (ESR)), radiographs (osteolyses) and a mini-incision biopsy (aspirate tissue for microbiological/histological examination) to identify periprosthetic infections according to Maurer et al. [17] and Laffer et al. [21]. After diagnosing a periprosthetic infection the further treatment is carried out with respect to following therapy algorithm (Figure 1 [Fig. 1]).
Figure 1: Algorithm “periprosthetic infection”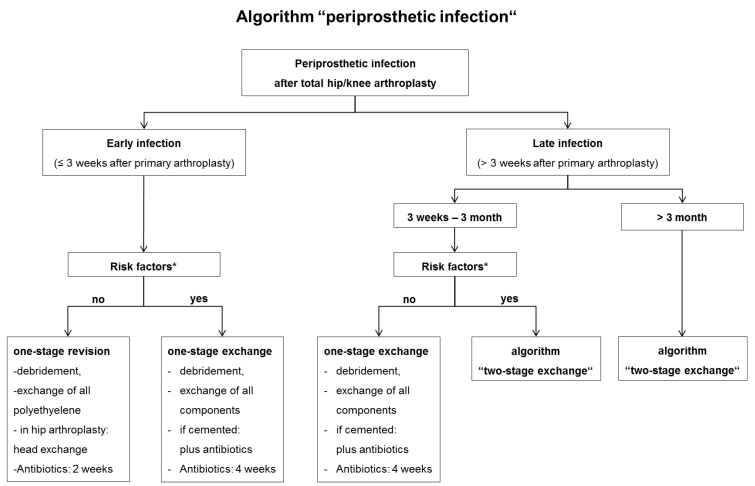
Early infection
In case of an early infection without risk factors at least the polyethylene component (hip, knee) and femoral head (hip) should be changed to enable cleaning of the metal-back surfaces by antiseptics (e.g. Octenisept®, Schülke und Mayr GmbH, Norderstedt, Germany) and pulsatile lavage. A complete one-stage exchange is indicated when risk factors are present (Table 1 [Tab. 1], Table 2 [Tab. 2]). From our point of view implant loosening, significant soft-tissue damage, and complicated-to-treat bacterial colonization as “absolute” are certain risk factors meaning that each of them requires at least a complete one-stage exchange. In individual cases even a two-stage exchange may be indicated. The risk factors we refer to are consistent with the Cierny-Mader criteria which are originally part of an osteomyelitis staging system [11], [22], [23]. Since these factors are known to impair wound healing and to encourage periprosthetic infections we rate them as “relative” factors. In absence of any “absolute” risk factors the orthopaedic surgeon should assess the individual “relative” risk profile of the patient in order to decide whether a partial or complete exchange is preferable. With multiple and/or very severe “relative” risk factors (local and systemic) even a two-stage exchange may be considered. If a cemented component fixation is necessary, antibiotics should be added to the cement adapted to the microbiological results. Even in case of negative or missing previous microbiological status the use of revision cement containing certain antibiotics (e.g. gentamycin, vancomycin and clindamycin) is recommended.
Table 1: Risk factors for endoprosthesis-related infections
Table 2: Relative risk factors for endoprosthesis-related infections according to Cierny-Mader [22, 23].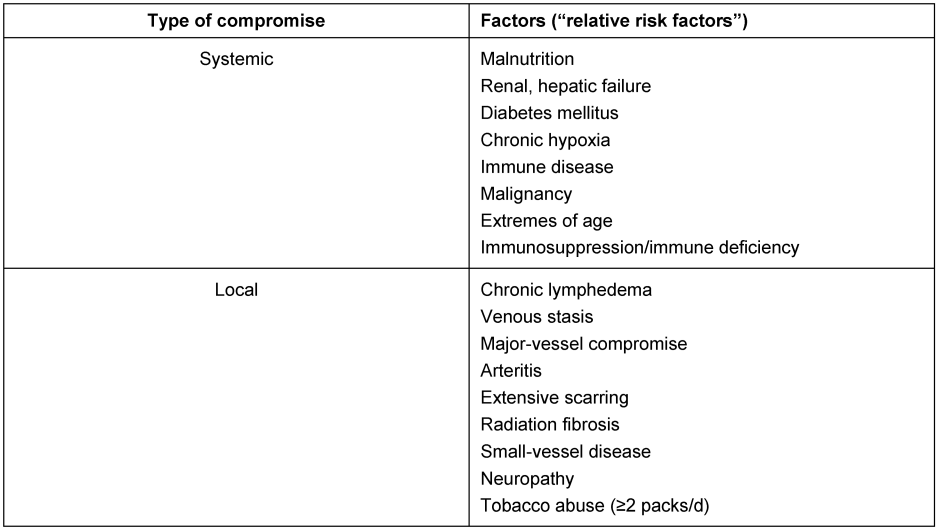
Moreover, any one-stage revision should include an exchange of gloves, instruments and sterile covering after pulsatile lavage. Tissue samples for microbiological and histological analyses are obtained intraoperatively in a standardized manner (Table 3 [Tab. 3]) in order to adapt the postoperative double antibiotic treatment. Without clinical, histological and microbiological sign of an infection after revision surgery i.v. antibiotics are administered for 2 weeks, otherwise for 4 weeks.
Table 3: Standardized extraction of tissue samples for microbiological and histological analysis [51] in the course of the revision operation including the explantation of the total hip/knee endoprosthesis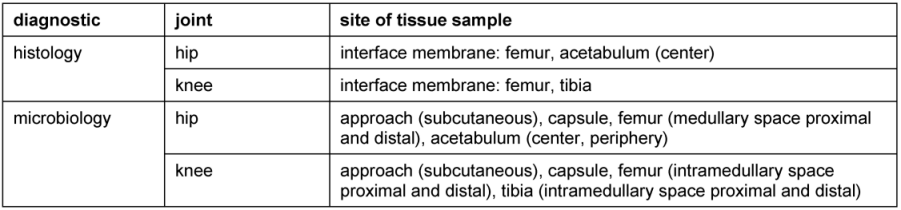
Late infection
Within the early period of the late periprosthetic infection (3 weeks to 3 month after arthroplasty) in absence of any risk factors a complete one-stage exchange is justified (Figure 1 [Fig. 1]). Otherwise we conduct a two-stage revision with a 6 weeks temporary spacer interval (Figure 2 [Fig. 2]). Handmade or ready-made antibiotic loaded cement spacers are widely accepted. Hip stems with an antimicrobial TiCuN-coating can also be used for selected patients. Regardless of the chosen type of spacer it must maintain the length of the soft-tissue and stabilize the affected joint. Application of an external orthosis may provide additional joint stability. Tissue samples for microbiological and histological analyses are obtained intraoperatively (Table 3 [Tab. 3]). The explanted endoprosthetic implants are additionally analyzed by a sonification culture in case of low-grade infection or negative previous microbiology despite clear infection signs according to Maurer et al. [17]. The double antibiotic treatment is adapted to the antibiogram and administered for 4 weeks (2 weeks i.v., 2 weeks p.o.).
Figure 2: Algorithm “two-stage revision”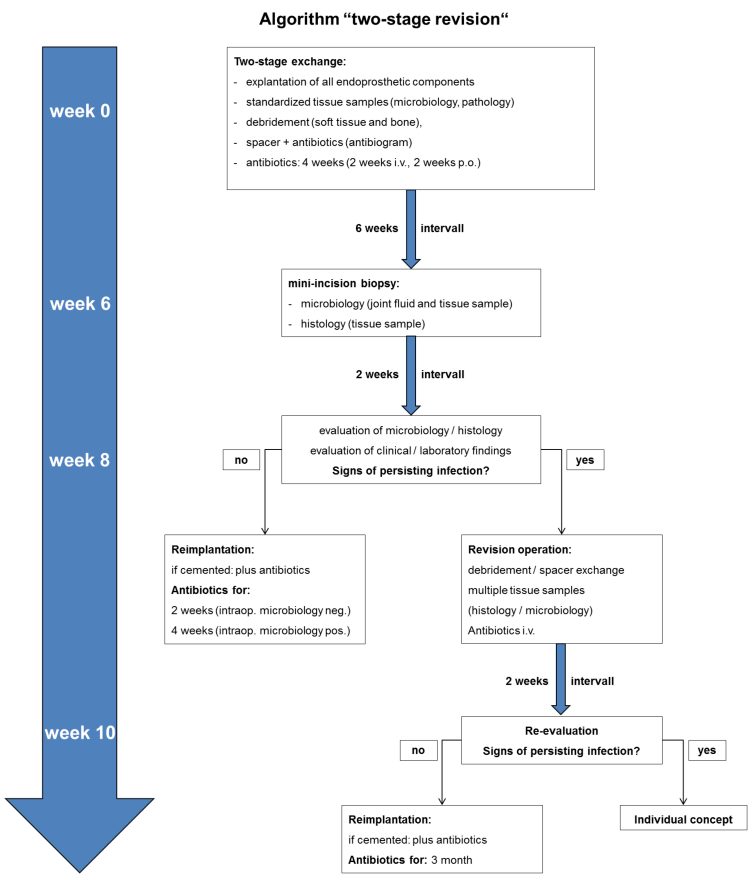
Joint fluid (cytology) and tissue samples (microbiology, histology) are obtained 6 weeks postoperatively. After 2 weeks incubation the results are evaluated along with the clinical and laboratory findings. Especially the ESR and CrP should not be significantly elevated.
If there are no signs of infection, 8 weeks after implant removal the revision endoprosthesis can be implanted accompanied by antibiotic treatment for 2 weeks (intraoperative microbiology negative or 4 weeks (intraoperative microbiology positive). Thereby multiple samples (two for microbiology and one for pathology) are taken from the periarticular tissue and intramedullary space. Otherwise in case of any signs of infection the antibiotic treatment is prolonged from two to four weeks.
In case of persisting signs of infection 8 weeks after implantation of the spacer, a revision operation including debridement and spacer exchange is indicated (Figure 2 [Fig. 2]). Tissue samples are taken in a standardized manner (Table 3 [Tab. 3]) to acquire a full-field analysis of the present microbiological colonization. After 2 weeks it must be decided whether re-implantation is possible or an individual concept is needed for the patient.
Discussion
In case of a necessary revision surgery of total joint arthroplasty it is crucial to distinguish between aseptic or septic revision because treatment is completely different. The diagnosis “periprosthetic infection” may be clear when purulent secretion and signs of septicaemia are apparent. But in cases of suspected septic loosening the preoperative laboratory and puncture fail to identify bacterial colonization in up to 20% of all cases [15], [20], [24], [25]. A mini-incision biopsy (e.g. automatic needle) obtaining tissue for a permanent histology is recommended to increase diagnostic sensitivity [26]. Particularly the microorganisms causing low-grade infections could be failed to identify by culture [27]. Aiming to acquire the complete spectrum of microorganism causing septic loosening especially for biofilm-producing bacteria or in case of previous antibiotic treatment sonification and 16S PCR of explanted devices have to be conducted. PCR and culturing the sonification fluid is likely to raise sensitivity of cultures significantly, but possibly wrong-positive results have to be critically evaluated [28], [29], [30].
The treatment of periprosthetic infections is undisputed in case of peracute respectively high-grade infection [31]. An urgent revision surgery with evacuation of pus is indicated. After gaining the tissue samples a calculated antibiotic therapy is initiated and should be adapted after receiving the microbiological results. Septic or multimorbid patients may need a two-stage explantation. Loose and easy removable implant parts as well as necrotic tissue are removed within the first operation, remaining cement or well-integrated implant parts are explanted after non-surgical stabilization of the patient.
In patients with early infection a one-stage procedure is widely accepted [6], [10], [12], [13]. Since most patients with an early infection lack significant risk factors due to the directly preceding arthroplasty, a one-stage partial exchange is sufficient. Some authors recommend a negative pressure therapy (NPT) (V.A.C.®, KCI Wiesbaden, Germany) combined with a suction/irrigation system [32], [33]. But due to the limited success rates of 50% to 60% [15], [32] the vacuum assisted closure therapy remains an option for isolated cases [13], [34].
But how patients with non-fulminant periprosthetic late infection should be treated is still discussed in literature controversially. One-stage as well as two-stage revisions have been reported to be successful in 80% to 100% [3], [15], [18], [35], [36]. One-stage concepts are described to be advantageous concerning duration of hospitalization, functional outcome, and economic aspects [15], [18], [24], [37]. But most of the proponents retract from their one-stage concept, whenever the bacterial colonization is unclear or not easy to treat, the patient`s general condition is reduced, soft-tissue is compromised or if the patient had multiple previous operations [3], [15], [18], [37]. Though this leads to a selection of patients, De Man et al. compared 22 patients after one-stage exchange to 50 patients after two-stage exchange. Although the one-stage patients had better prerequisites, the clinical (Harris hip score) and radiological outcome did not differ after 2 years between the two groups [18]. Since the vast majority of the patients meet the risk factors given above, we agree that in case of late infections the two-stage revision is still the “gold standard” [3], [4], [10], [12], [13], [38]. A complete removal of the colonized implants and a thorough debridement of infected tissue is the common basis for both concepts.
The recommended spacer interval varies markedly in the literature from 3 weeks to >1 year, most common are 6–12 weeks [3], [10], [17]. Our two-stage protocol is based on an 8 week interval providing a 2 week antibiotic-free period before obtaining tissue samples. Due to possible residual effects of systemic and local antibiotics tissue samples are proven to increase the diagnostic sensitivity for persisting infection [20], [39], [40], [41]. As standard spacers we are using antibiotic loaded cement spacers for the knee and hip as well as TiCuN coated spacers (hip) for selected cases. However, cement spacers, especially articulating spacers, are suspected to produce wear debris leading to higher wear rates of the revision implant [3]. As an alternative, cement without zirconium dioxide particles (Copal® spacem, Heraeus Medical, Wehrheim, Germany) or hip hemi-arthroplasties with antimicrobial coating may be used in the future [42], [43], [44].
The reimplantation is performed 8 weeks after septic explantation if the preoperatively obtained tissue shows no microbiological or histological signs of infection. Furthermore, no clinical/laboratory infection signs are apparent (Figure 2 [Fig. 2]). Hence the validity of laboratory parameters for diagnosing a periprosthetic infection is not entirely clarified so far [20], [45], [46], [47], [48], [49]. In particular, the ESR and CRP should be within normal range or is only slightly elevated (e.g. CRP ≤12 mg/L) [48]. The type of revision endoprosthesis (length, modularity, constrained knee, cemented/cementless fixation) depends on the quality of the bone, the type of bone defect and the condition of soft tissue structures. For cemented implants the antibiotics added to the bone cement can be adapted to the sensitivity of the bacteria [15], [17], [18].
In case of persisting infection another kind of revision surgery is indicated (Figure 2 [Fig. 2]) [9], [13], [17]. Patients with not controllable infection that are multimorbid, limited operable or have a short life expectancy may need an individual concept. Alternative treatment options comprise Girdlestone hip, knee arthrodesis, permanent fistula or amputation as a last resort [9], [15], [50].
Conclusions
Treatment of implant-associated infections after total hip and knee arthroplasty requires an experienced hospital staff and appropriate facilities (e.g. operation rooms, isolation sick chambers, intensive care unit). Moreover a wide spectrum of diagnostic (e.g. microbiology, pathology, radiology, nuclear medicine) and therapeutic possibilities (multidisciplinary physician team, individual implants, psychological support) should be provided. A treatment algorithm makes the necessary steps and decision pathways more comprehensible for the patient and new staff. The presented algorithm has been thoroughly refined with regard to the literature and the experience of specialized orthopaedic surgeons from several university and regional hospitals in Mecklenburg-Pomerania, Germany. Standardized documents (e.g. patient information sheet, study agreement) should avoid to loose important information (e.g. “mismatch” of implants, individual concepts, recurrence of infection) from the therapeutic and forensic aspect. A quality management system can help to define important aspects of the outpatient treatment such as screening for MRSA, wound care and radiological/clinical follow-up.
Notes
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
For collaboration within the BMBF project “HiCare” and their contribution to “AG Endoprothesen-assoziierte Infektion” we would like to thank (in alphabetical order):
Dr. H. Büchner (Heraeus Medical GmbH, Wehrheim), Prof. Dr. A. Erbersbobler (Institute of Pathology, University Rostock), Dr. D. Flachsmeyer (Medical Center Südstadt, Rostock), Dr. D. Ganzer (Medical Center Altentreptow), Dr. S. Harder (Medical Center Güstrow), Dr. N. Hübner (Institute of Hygiene and Environmental Medicine, University Greifswald), Dr. R. Kasch (Department of Orthopaedics, University Greifswald), Dr.-Ing. D. Kluess (ForBioMit, University Rostock), Prof. Dr. A. Kramer (Institute of Hygiene and Environmental Medicine, University Greifswald), Dr. H. Michelsen (Hospital Bad Doberan), Prof. Dr. A. Podbielski (Institute of Medical Mikrobiology, University Rostock), Prof. Dr. F. Prall (Institute of Pathology, University Rostock), Dr. C. Prinz (DOT GmbH Rostock), Dr. H. Scheerat (Hospital Grimmen), Dr. H.H. Springer (Medical Center Schwerin), PD Dr. D. Stengel (Trauma Center Berlin), PD Dr. M. Witt (Rostock).
This work is supported by the Federal Ministry of Education and Research (BMBF) and the Ministry of Education, Science and Culture of the state Mecklenburg-Pomerania.
References
[1] BQS-Bundesauswertung 2008. Knie-Totalendoprothesen-Erstimplantation. BQS Bundesgeschäftsstelle Qualitätssicherung gGmbH; 2009. p. 1-63. Available from: http://www.bqs-outcome.de/2008/ergebnisse/leistungsbereiche/knie_tep_erst/index_html[2] BQS-Bundesauswertung 2008. Hüft-Endoprothesen-Erstimplantation; 2009. p. 1-65. Available from: http://www.bqs-outcome.de/2008/ergebnisse/leistungsbereiche/hueft_endo_erst/index_html
[3] Fink B. Revision of late periprosthetic infections of total hip endoprostheses: pros and cons of different concepts. Int J Med Sci. 2009;6(5):287-95. Available from: http://www.medsci.org/v06p0287.htm
[4] Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645-54. DOI: 10.1056/NEJMra040181
[5] Bengtson S, Knutson K. The infected knee arthroplasty. A 6-year follow-up of 357 cases. Acta Orthop Scand. 1991;62(4):301-11. DOI: 10.3109/17453679108994458
[6] Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111-22.
[7] Garellick G, Kärrholm J, Rogmark C, Herberts P. Swedish Hip Arthroplasty Register: Annual Report 2008. Department of Ortopaedics, Sahlgrenska University Hospital; October 2009. Available from: https://www.jru.orthop.gu.se/
[8] Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-8. DOI: 10.1097/01.blo.0000214439.95268.59
[9] Ruchholtz S, Täger G, Nast-Kolb D. Die infizierte Hüftgelenksendoprothese [The infected hip prosthesis]. Unfallchirurg. 2004;107(4):307-17. DOI: 10.1007/s00113-004-0751-9
[10] Fink B, Grossmann A, Fuerst M, Schäfer P, Frommelt L. Two-stage cementless revision of infected hip endoprostheses. Clin Orthop Relat Res. 2009;467(7):1848-58. DOI: 10.1007/s11999-008-0611-y
[11] Cierny G 3rd, DiPasquale D. Periprosthetic total joint infections: staging, treatment, and outcomes. Clin Orthop Relat Res. 2002;(403):23-8.
[12] Zimmerli W, Ochsner PE. Management of infection associated with prosthetic joints. Infection. 2003;31(2):99-108. DOI: 10.1007/s15010-002-3079-9
[13] Militz M, Bühren V. Wechsel infizierter Knie- und Hüftendoprothesen [Replacement of infected knee and hip endoprostheses ]. Der Chirurg. 2010;81(4):310-20. DOI: 10.1007/s00104-009-1842-5
[14] Nuryastuti T, Krom BP, Aman AT, Busscher HJ, van der Mei HC. Ica-expression and gentamicin susceptibility of Staphylococcus epidermidis biofilm on orthopedic implant biomaterials. J Biomed Mater Res A. 2011;96(2):365-71. DOI: 10.1002/jbm.a.32984
[15] Friesecke C, Wodtke J. Management des Protheseninfektes [Management of periprosthetic infection]. Chirurg. 2008;79(8):777-92. DOI: 10.1007/s00104-008-1570-2
[16] Walter G, Bühler M, Hoffmann R. Der zweizeitige septische Hüft-TEP-Wechsel beim periprothetischen Spatinfekt. Frühergebnisse nach Implantation einer modularen reversen Hybridprothese [Two-stage procedure to exchange septic total hip arthroplasties with late periprosthetic infection. Early results after implantation of a reverse modular hybrid endoprosthesis]. Unfallchirurg. 2007;110(6):537-46. DOI: 10.1007/s00113-007-1238-2
[17] Maurer TB, Ochsner PE. Infekt nach Knietotalprothesenimplantation. Zweizeitiger Wechsel als Element des Liestaler Behandlungsalgorithmus [Infected knee arthroplasty. A treatment algorithm at the Kantonsspital Liestal, Switzerland]. Orthopäde. 2006;35(9):917-8, 920-8. DOI: 10.1007/s00132-006-0978-y
[18] De Man FH, Sendi P, Zimmerli W, Maurer TB, Ochsner PE, Ilchmann T. Infectiological, functional, and radiographic outcome after revision for prosthetic hip infection according to a strict algorithm. Acta Orthop. 2011;82(1):27-34. DOI: 10.3109/17453674.2010.548025
[19] Winkler H, Stoiber A, Kaudela K, Winter F, Menschik F. One stage uncemented revision of infected total hip replacement using cancellous allograft bone impregnated with antibiotics. J Bone Joint Surg Br. 2008;90(12):1580-4. DOI: 10.1302/0301-620X.90B12.20742
[20] Gollwitzer H, Diehl P, Gerdesmeyer L, Mittelmeier W. Diagnostische Strategien bei Verdacht auf periprothetische Infektion einer Kniegelenktotalendoprothese. Literaturubersicht und aktuelle Empfehlungen [Diagnostic strategies in cases of suspected periprosthetic infection of the knee. A review of the literature and current recommendations]. Orthopäde. 2006;35(9):904, 906-8, 910-6. DOI: 10.1007/s00132-006-0977-z
[21] Laffer RR, Graber P, Ochsner PE, Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect. 2006;12(5):433-9. DOI: 10.1111/j.1469-0691.2006.01378.x
[22] Mader JT, Shirtliff M, Calhoun JH. Staging and staging application in osteomyelitis. Clin Infect Dis. 1997;25(6):1303-9. DOI: 10.1086/516149
[23] Cierny G, Mader JT, Pennick H. A clinical staging system of adult osteomyelitis. Contemp Orthop. 1985;10:17-37.
[24] Steinbrink K, Frommelt L. Behandlung der periprothetischen Infektion der Hüfte durch einzeitige Austauschoperation [Treatment of periprosthetic infection of the hip using one-stage exchange surgery]. Orthopäde. 1995;24:335-43.
[25] Taylor T, Beggs I. Fine needle aspiration in infected hip replacements. Clin Radiol. 1995;50(3):149-52. DOI: 10.1016/S0009-9260(05)83044-2
[26] Lonner JH, Desai P, Dicesare PE, Steiner G, Zuckerman JD. The reliability of analysis of intraoperative frozen sections for identifying active infection during revision hip or knee arthroplasty. J Bone Joint Surg Am. 1996;78(10):1553-8.
[27] Pandey R, Drakoulakis E, Athanasou NA. An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J Clin Pathol. 1999;52(2):118-23. DOI: 10.1136/jcp.52.2.118
[28] Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654-63. DOI: 10.1056/NEJMoa061588
[29] Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999;37(10):3281-90.
[30] Nelson CL, McLaren AC, McLaren SG, Johnson JW, Smeltzer MS. Is aseptic loosening truly aseptic? Clin Orthop Relat Res. 2005;(437):25-30. DOI: 10.1097/01.blo.0000175715.68624.3d
[31] Krenn V, Morawietz L, Jakobs M, Kienapfel H, Ascherl R, Bause L, Kuhn H, Matziolis G, Skutek M, Gehrke T. Gelenkendoprothesenpathologie. Histopathologische Diagnostik und Klassifikation [Joint endoprosthesis pathology. Histopathological diagnostics and classification]. Pathologe. 2011;32(3):210-9. DOI: 10.1007/s00292-011-1418-2
[32] Lehner B, Suda A, Zeifang F. Ergebnisse der V.A.C.-Instill-Therapie bei Infekten von Knie- und Hüftendoprothesen. In: Foederatio Medicorum Chirurgicorum Helvetica, ed. V.A.C.Therapy – Better woundcare by any definition. Luzern: 2007. p. 44-8.
[33] Steinbrink K, Mella-Schmidt C. Stellenwert der Spül-Saugdrainage bei der Behandlung des Frühinfekts von Gelenkimplantaten. Chirurg. 1989;60(11):791-4.
[34] Seifert J, Ekkernkamp A. Vakuumtherapie: Chancen und Grenzen [Vacuum assisted closure technique (VACT): indications and contraindications]. GMS Krankenhhyg Interdiszip. 2009;4(2) Doc13. DOI: 10.3205/dgkh000138
[35] Lai KA, Shen WJ, Yang CY, Lin RM, Lin CJ, Jou IM. Two-stage cementless revision THR after infection. 5 recurrences in 40 cases followed 2.5-7 years. Acta Orthop Scand. 1996;67(4):325-8. DOI: 10.3109/17453679609002324
[36] Younger AS, Duncan CP, Masri BA. Treatment of infection associated with segmental bone loss in the proximal part of the femur in two stages with use of an antibiotic-loaded interval prosthesis. J Bone Joint Surg Am. 1998;80(1):60-9.
[37] Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63-B(3):342-53.
[38] Sukeik M, Haddad FS. Two-stage procedure in the treatment of late chronic hip infections – spacer implantation. Int J Med Sci. 2009;6(5):253-7.
[39] Barrack RL, Jennings RW, Wolfe MW, Bertot AJ. The Coventry Award. The value of preoperative aspiration before total knee revision. Clin Orthop Relat Res. 1997;(345):8-16.
[40] Fuerst M, Fink B, Rüther W. Die Wertigkeit von praoperativer Punktion und arthroskopischer Synovialisprobenentnahme bei Knietotalendoprothesenwechsel [The value of preoperative knee aspiration and arthroscopic biopsy in revision total knee arthroplasty]. Z Orthop Ihre Grenzgeb. 2005;143(1):36-41. DOI: 10.1055/s-2004-836252
[41] Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82-A(11):1552-7.
[42] Haenle M, Fritsche A, Zietz C, Bader R, Heidenau F, Mittelmeier W, Gollwitzer H. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: in vitro effectiveness against MRSA and mechanical properties. J Mater Sci Mater Med. 2011;22(2):381-7. DOI: 10.1007/s10856-010-4204-4
[43] Gollwitzer H, Ibrahim K, Meyer H, Mittelmeier W, Busch R, Stemberger A. Antibacterial poly (D,L-lactic acid) coating of medical implants using a biodegradable drug delivery technology. J Antimicrob Chemother. 2003;51(3):585-91. DOI: 10.1093/jac/dkg105
[44] Heidenau F, Mittelmeier W, Detsch R, Haenle M, Stenzel F, Ziegler G, Gollwitzer H. A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J Mater Sci Mater Med. 2005;16(10):883-8. DOI: 10.1007/s10856-005-4422-3
[45] Hsieh PH, Chen LH, Chen CH, Lee MS, Yang WE, Shih CH. Two-stage revision hip arthroplasty for infection with a custom-made, antibiotic-loaded, cement prosthesis as an interim spacer. J Trauma. 2004;56(6):1247-52. DOI: 10.1097/01.TA.0000130757.53559.BF
[46] Austin MS, Ghanem E, Joshi A, Lindsay A, Parvizi J. A simple, cost-effective screening protocol to rule out periprosthetic infection. J Arthroplasty. 2008;23(1):65-8. DOI: 10.1016/j.arth.2007.09.005
[47] Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621-6. DOI: 10.1007/s00264-010-1175-5
[48] Parvizi J, Suh DH, Jafari SM, Mullan A, Purtill JJ. Aseptic loosening of total hip arthroplasty: infection always should be ruled out. Clin Orthop Relat Res. 2011;469(5):1401-5. DOI: 10.1007/s11999-011-1822-1
[49] Drago L, Vassena C, Dozio E, Corsi MM, De Vecchi E, Mattina R, Romanò C. Procalcitonin, C-reactive protein, interleukin-6, and soluble intercellular adhesion molecule-1 as markers of postoperative orthopaedic joint prosthesis infections. Int J Immunopathol Pharmacol. 2011;24(2):433-40.
[50] Tiemann AH, Homagk L, Diefenbeck M, Mückley T, Hofmann GO. Hüftprothesenerhalt mit lokaler chirurgischer Revision und Anlage einer Fistula persistens: Ausnahmeoption zur palliativen Therapie des periprothetischen Infekts bei alten polymorbiden Patienten? [Preservation of hip prosthesis with local surgical revision and creation of a fistula persistens: an option for palliative treatment of periprosthetic infection in old, polymorbid patients?]. Unfallchirurg. 2007;110(12):1021-9. DOI: 10.1007/s00113-007-1367-7
[51] Morawietz L, Classen RA, Schröder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, Otto M, Barden B, Aigner T, Stiehl P, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader CP, Kirschner S, Lintner F, Rüther W, Bos I, Hendrich C, Kriegsmann J, Krenn V. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59(6):591-7. DOI: 10.1136/jcp.2005.027458




