[Diagnosis, hearing care and early intervention in children with hearing loss after the newborn hearing screening – does all this happen really “early”?]
Karolin Schäfer 1Manfred Hintermair 2
1 University of Cologne, Germany
2 Heidelberg University of Education, Heidelberg, Germany
Abstract
Background: Universal Newborn Hearing Screening (UNHS) in Germany offers the opportunity to detect congenital hearing loss at the earliest possible time and to provide early diagnosis, early hearing device provision, and early intervention to children with peripheral hearing loss. However, hearing screening alone does not necessarily result in children receiving hearing devices and early intervention at the earliest.
Method: In the present study, n=316 mothers of children with hearing loss born in 2009 or later provided retrospective information on a questionnaire on if their child had undergone UNHS, at what time the final diagnosis was made, and at what time hearing devices were provided and early intervention began. For evaluation, cluster analyses, discriminant analyses, and variance analyses were performed.
Results: The results show that particularly children with congenital unilateral and mild hearing loss were recognized later. Severe-to-profound congenital hearing loss is diagnosed and treated earlier, but not in all cases. As expected, children with late-onset hearing loss are diagnosed much later than children with congenital hearing loss. There are large time differences between the time of diagnosis, the provision of hearing devices and the onset of early intervention between the three groups.
Conclusion: Early detection of congenital and late-onset hearing loss in childhood is a prerequisite for early provision of hearing devices and referral to early intervention. In addition to improving tracking after UNHS, there is a need for further hearing screenings and pediatric audiometric or hearing diagnostics.
Keywords
(Universal) Newborn Hearing Screening, early detection, hearing loss, children
Introduction
The introduction of a nationwide Universal Newborn Hearing Screening (UNHS) resulted in children with significant hearing loss being identified and provided with hearing technology and educational support much earlier than before the introduction of the UNHS [1], [2], [3].
Accordingly, the developmental outcomes of children with hearing loss born after the introduction of the UNHS are encouraging. This can be seen in a series of studies documenting the impact of early identification of hearing loss on different areas of child development [4], [5], [6]. A well-known study in this context is the Australian longitudinal study LOCHI, in which 470 children with an early-detected hearing loss were examined at multiple time points using an extensive battery of developmental tests [7], [8], [9]. Ching et al. [8] presented a summary of the results at the time when the participating children were five years old. The results show a strong positive effect of early identification and early intervention on language development (both expressive and receptive), especially in the children who were fitted with hearing aids or cochlear implants (CIs) at an early age. Lederberg et al. [10] also confirm this for the development of sign language. The LOCHI study also found significant correlations between early identification and early intervention and improvements in non-verbal cognitive abilities and psychosocial skills for psychosocial and cognitive development [8].
However, it must be noted that the developmental outcomes of children with hearing loss continue to show a wide range of variation, i.e. the individual developmental progress of children with hearing loss can be very different [11]. Developmental delays of speech perception and processing, which cannot be ruled out even if hearing loss is diagnosed early, may influence a number of developmental processes that are important for age-appropriate development. The special perceptual situation of children with hearing loss therefore requires specific measures in education and upbringing, even after the introduction of the UNHS [11], [12]. However, it is undisputed that an early diagnosis of hearing loss enables better overall developmental and educational opportunities for children with hearing loss than there were before the introduction of the UNHS.
This makes it all the more important to address potential problem areas associated with the UNHS in order to further optimize the opportunities that the UNHS offers. In this context, it is also important to face the challenges that need to be overcome in the detection of hearing loss that go beyond the application of the UNHS. For example, von Nennstiel et al. [2] noted that a significant proportion of children with hearing loss detected through the UNHS still cannot be followed up (see also [1]). In addition to children who do not present for follow-up examinations (“lost-to-follow-up”), there are also children with mild unilateral or bilateral hearing loss who are not detected by the transient evoked otoacoustic emissions (TEOAE) screening method and run the risk of being diagnosed much later than children with moderate to severe or profound hearing loss [13], which in turn results in late provision of hearing hearing devices and early intervention. However, this may also be related to uncertainty on the part of both professionals and parents about the potential benefits of (early) support for this group of children with mild hearing loss [14]. The long-term negative consequences of late-onset of hearing care and support for mild hearing loss are well documented by now [5]. Van de Sand et al. [15] found in their analysis of the prevalence of peripheral hearing loss based on routine data from statutory health insurance companies (SHI) that even after the nationwide introduction of UNHS in 2009, a significant proportion of children with hearing loss do not receive hearing aids up to the age of four to six. For this analysis, the 2010 birth cohort was analyzed throughout Germany for 10 years; further examinations of children born later are desirable.
The results presented below are based on selected data from a survey of mothers of children with hearing loss in Germany who had undergone UNHS. The survey also collected information on time of diagnosis and onset of hearing device use and early intervention.
The research question for this study is whether there are differences in the final age of diagnosis, the onset of first hearing device provision, and the onset of early intervention in children who have undergone UNHS.
Methodology
Study conduct
The data was collected as part of an (optional) online and paper-pencil survey of mothers of children with peripheral hearing loss from March to May 2023. For this purpose, six special schools for the deaf and hard of hearing, two Cochlear Implant Centers, and three early intervention centers from three German federal states (Baden-Württemberg, Bavaria, North Rhine-Westphalia) were contacted. They were asked to send the invitation letter to the survey with the link to the online questionnaire by email to all families with a child with hearing loss from pre-school age (in special schools as well as in inclusive schools). The data from the paper-pencil questionnaires was entered into the online tool afterwards.
After completion of the online survey, n=541 questionnaires were downloaded from the server. According to the institutions involved in the survey, approx. 2,241 families were informed about the survey (response rate: approx. 24.1%).
Of the n=541 questionnaires, n=316 were included in the analysis. The others were discarded because either the information was incomplete (n=115) or because the children were born before 2009 and it was therefore not possible to ensure whether an UNHS had been performed (n=100). A further n=10 questionnaires were removed from the study because the mothers stated (with reasons) that their children were not enrolled in UNHS. This means that (according to the mothers’ statements) 3.1% of the sample did not undergo UNHS.
Sample
Table 1 [Tab. 1] shows the information provided by the mothers on the socio-demographic and hearing loss-specific characteristics surveyed, including school education (highest educational qualification).
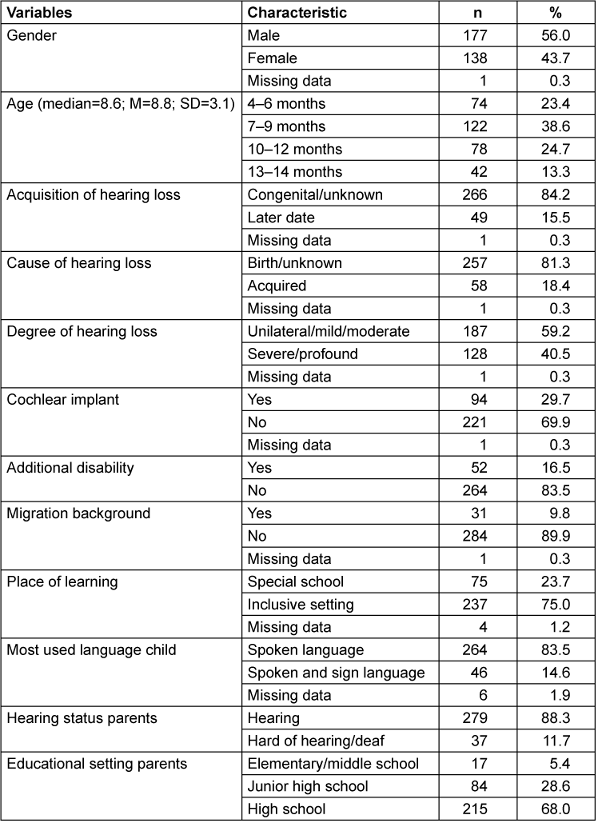
With regard to the information provided by the mothers on the time of acquisition of the hearing loss, it should also be mentioned that, in addition to the categories “congenital/unknown” and “acquired”, further aspects were queried that provided additional information on the time of acquisition of hearing loss, such as the diagnosis. If a diagnosis clearly indicated an acquired hearing loss (e.g. cytomegaly, meningitis), the information provided by the parents was confirmed. In the case of unclear or missing statements and for children who were detected and provided at a very young age, the time of acquisition of hearing loss was assigned to the “unknown” category, even if parents had previously selected the “acquired” category.
Assessment instrument
The aspects from the overall questionnaire package relevant to this article include information on socio-demographic and hearing loss-specific characteristics of the children and their families (see Table 1 [Tab. 1]) as well as statements made by the mothers specifically related to the UNHS. They were asked whether children underwent UNHS. They were informed that there is an entry on the UNHS in the yellow “U-Heft” for statutory examinations for early detection of diseases in infants by the pediatrician. If the parents stated that their child had not undergone NHS, they were asked why. This was followed by questions about the age of the child in years and months when the hearing loss was finally identified (diagnosed), about the first provision of hearing devices, and whether and when early intervention began.
The statistical analysis of the data was performed using IBM SPSS Statistics 27.
Results
Grouping of children with similar characteristics
In the first step of the analysis, an attempt was made to form groups of children with hearing loss with the most similar characteristics possible. For this, a two-step cluster analysis was performed in which, in addition to the 12 socio-demographic and hearing loss-specific variables (see Table 1 [Tab. 1]), a categorized variable “time of first hearing device provision” (“provided earlier” (≤12 months (n=127; 40.2%) versus “provided later” (>12 months (n=189; 59.8%) was included.
Clustering into three groups with the inclusion of n=303 of the total n=316 children proved to be the best solution in terms of content. The results of the analysis are shown in Table 2 [Tab. 2]. The cluster quality was in the medium to upper range (silhouette coefficient=0.4), the cluster size ratio of 2.56 is acceptable.
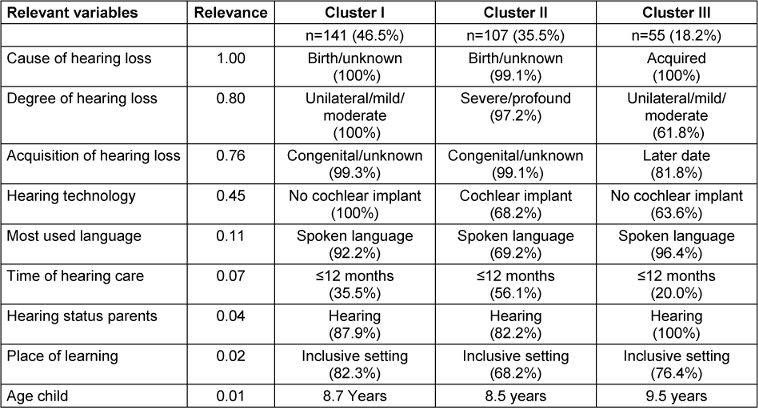
Nine of the 13 variables included significantly contributed to cluster formation (p≤.05); the variables of gender, additional needs, migration background, and parents’ educational level did not contribute to the distinctiveness of the three clusters (p>.05).
Cluster I: Cluster I was the largest and comprised almost half of the children (n=141, 46.5%). It combined children for whom the cause or acquisition of the hearing loss was exclusively unknown/congenital/genetic and who all had a unilateral, mild, or moderate hearing loss. None of the children in this cluster had a cochlear implant, more than 90 percent of the children used spoken language and more than 80 percent of the children were studying in inclusive school settings. Around a third of the children were fitted with hearing devices within the first year of life, two thirds of the children in this cluster were fitted later.
Cluster II: This was the second largest cluster (n=107, 35.5%). As with the children in Cluster I, the acquisition or cause of the hearing loss was almost completely unknown/congenital/genetic. The main difference to Cluster I is that almost all children in Cluster II had a severe-to-profound hearing loss or residual hearing. As a result, two thirds of the children in this cluster were fitted with at least one cochlear implant, and almost a third of the children used spoken and sign language. Also, almost a third of the children in this cluster attended a special school. In addition, there were significantly more children in this cluster whose parents also had a hearing loss. In contrast to Cluster I, significantly more children were provided with hearing devices in the first year of life (presumably due to the higher degree of hearing loss).
Cluster III: This was the smallest cluster (n=55, 18.2%). It included children with (mostly later) acquired hearing loss. This is also reflected in the fact that 80% of the children received hearing devices only after the age of one year. This cluster included children with mild and moderate hearing loss as well as children with severe-to-profound hearing loss. The hearing technology used (both CI and hearing aids) varied accordingly. The communication modality was almost exclusively spoken language, and inclusive schooling was also predominant. All parents had normal hearing. With around nine and a half years of age, the children in this cluster were on average one year older than the children in clusters I and II.
The grouping made by the cluster analysis was additionally checked by a discriminant analysis, which can be used to test whether the variables located in the clusters with their focal points actually contributed best to the differentiation of the three groups. Using the relevant statistical parameters (eigenvalues, canonical correlations, Wilks’ lambda test, Chi2), the results confirmed the three clusters formed. 98.7 percent of the cases grouped by the cluster analysis were correctly classified by the discriminant analysis. This was not the case for four out of 303 cases (1.3%).
Hearing care-relevant aspects in the three clusters
The children with hearing loss in the three clusters were then compared with regard to the time of the final diagnosis, onset of hearing device use, and onset of early intervention. For this, a one-way analysis of variance (ANOVA) was performed with the age of diagnosis, the age at first hearing device use, and the age at onset of early intervention (in months) as dependent variables and cluster membership as an independent factor. Table 3 [Tab. 3] shows the results. The lower number of children in the variable “hearing care” was due to the fact that some of the unilateral or mild hearing loss cases were obviously not provided with hearing devices according to the mothers. The lower number of children for the variable “onset of early intervention” was due to the fact that some children were already too old for early intervention due to the late onset of the hearing loss.
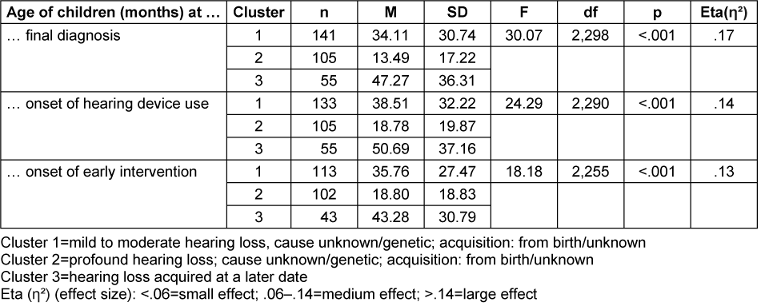
There were highly significant differences (p<.001) with medium to high effect sizes (partial ƞ2 between .13 and .17) between the three clusters for all dependent variables examined. The post-hoc tests between the three clusters were also significant for all comparisons made (at least p=.028), with one exception: with regard to the onset of early intervention, cluster 1 and cluster 3 do not differ significantly from each other (p=.217).
The results of the ANOVA showed that time of diagnosis, onset of hearing device use, and onset of early intervention were significantly earlier for children with a severe congenital hearing loss (cluster 2) than for the other two clusters. Clusters 1 and 3 also differed in terms of age of diagnosis and onset of hearing device use, albeit to a lesser extent. The children with (mostly later) acquired hearing loss were (as expected) the oldest in the areas examined.
Deepening the content of the cluster differences
To expand on the results presented in Table 3 [Tab. 3], the descriptive data in Table 4 [Tab. 4] show in which time frames how many children with hearing loss in the individual clusters were diagnosed and provided with hearing aids and early intervention (figures in percent).
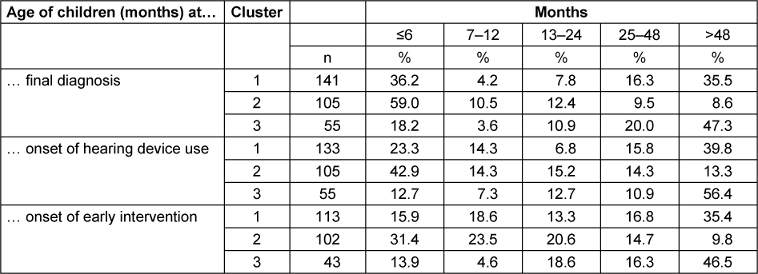
In terms of the time of final diagnosis, 69.5% of children in Cluster 2, which included all children with severe-to-profound congenital hearing loss, were diagnosed within the first year of life, compared to 40.4% of children in Cluster 1 who all had unilateral or mild to moderate hearing loss. More than half of the children in cluster 1 (51.8%) were diagnosed after the second year of life (35.5% of them after the fourth year of life), while only 18.1% of children in cluster 2 were diagnosed after the second year of life. In children from cluster 3, whose hearing loss was acquired or not congenital, 67.3% were diagnosed (as expected) after the second year of life, 47.3% of them after the 4th year of life.
With regard to onset of hearing device use, which is of particular importance for hearing and speech development from an audiological perspective, it can be seen that 57.2 percent of children with severe-to-profound hearing loss from birth in cluster 2 received their first hearing device within the first year of life. 42.8 percent of children in this cluster received their first hearing device after the first year of life. For children in cluster 1, the number of children who received hearing services within the first year of life (37.6%) was roughly the same as the number of children who were diagnosed at this time (40.4%). However, the onset of hearing device use in these children shifted more towards the second half of the first year of life. The number of children in this cluster who received a hearing device after the age of two (55.6%) was similar to the number of children who were also diagnosed during at this time (51.8%). Among the children in cluster 3 whose hearing loss was acquired or not congenital, the proportion of children who were provided with hearing devices for the first time after the age of two (67.3%) was identical to the age of diagnosis (67.3%).
With regard to the onset of early intervention, which is particularly important from an educational point of view, 54.9 percent of children in cluster 2 received early intervention in the first year of life, while 20.6 percent of children started early intervention in the second year of life. Of the children in cluster 1, 34.5 percent received early intervention in the first year of life. The number of children from this cluster who received early intervention after the second year of life (or after the age of 4) (52.2%) roughly corresponds to the age at diagnosis (51.8%) and the age at onset of hearing device use (55.6%). For children from cluster 3 whose hearing loss was acquired or not congenital, the proportion of children who received early intervention after the age of two (62.8%) corresponds approximately to the age at diagnosis (67.3%) and the onset of first hearing device use (67.3%).
Discussion
The discussion of the results is subject to the fact that all the data collected is based on the information provided by the mothers of the children with hearing loss and thus the entire information on the age of diagnosis, the onset of hearing device use and the onset of early intervention is subject to the accuracy of the mothers’ memories [16], [17]. This should not be underestimated considering that the children surveyed were between four and 14 years old at the time of the interview and therefore the information asked for had dated back a long time. Information from parents about events that occurred a long time ago tends to be not as reliable as information about current events [18].
Taking this into account, the results of the cluster analysis show that three main groups of children with hearing loss with different characteristic structures can be identified in the context of the UNHS. The data on time of diagnosis, onset of hearing device use, and the onset of early intervention in these groups provide indications of specific diagnostic and educational challenges.
The largest group, accounting for almost half of the children in this study, are children with unilateral or mild to moderate hearing loss whose hearing loss is congenital according to their mothers [19]. Only around 40 percent of these children are finally diagnosed and provided with hearing devices within the first year of life, and even fewer receive early intervention during this period. In contrast, more than half of the children in this group are only diagnosed, provided with hearing devices and early intervention after the second year of life (with a clear focus after the age of 4). This fact is confirmed by studies on children with unilateral hearing loss [20] and children with mild hearing loss [21]. One of the reasons for this is that the UNHS cannot always reliably detect mild hearing loss in particular if the recording of otoacoustic emissions is the only screening method used. The study also documents how the development of children may be hindered by late detection and late onset of early intervention [7], [22].
The second largest group, comprising around one third of the children, are children with severe-to-profound hearing loss or residual hearing whose hearing loss is also congenital according to the mothers. In more than half of the children in this cluster, diagnosis, onset of hearing device use, and onset of early intervention take place within the first year of life. However, there are also more than 40 percent of children in this cluster for whom hearing device provision and the onset of early intervention only take place after the first year of life (in around a quarter of the children after the age of 2). Given the knowledge of the effects of untreated severe-to-profound hearing loss on a child’s development, this result should be seen as a mandate to optimize the hearing care processes [1].
Summarizing the results of the two groups, it can be stated that there are large differences of 17 to 20 months between the two groups in terms of time of diagnosis, onset of hearing device use and the onset of early intervention. This means that children with unilateral hearing loss and children with bilateral mild to moderate hearing loss are diagnosed, provided with hearing devices and early intervention around one and a half years later on average. No information is available on the reasons for this for the children examined in this study, but the results confirm findings from other studies that point to the necessity of improving hearing care provision for these children in particular [20], [21].
The third group comprises just under 20 percent of the entire sample and includes children who were not born with hearing loss but acquired hearing loss later. These are children who probably passed UNHS. Walker et al. [13], referring to data from other studies [23], [24], [25], state that this is the case for about seven to 25 percent of all children with hearing loss; they pass through the UNHS without positive findings and the hearing loss occurs and is diagnosed later. In the present study, the time of diagnosis, onset of hearing device use and the onset of early intervention took place after the age of two for over 60 percent of the children and after the age of four for around 50 percent of the children. This result clearly indicates that both parents and professionals cannot rest assured if the children pass UNHS. This means that hearing screening and diagnostic tests shall be carried out regularly even after passing UNHS [26].
Study limitations
The aforementioned problem of mothers’ memory accuracy means that the results presented in this study, and in particular the percentages given, can only serve as a rough guide. It is possible that after more than ten years, parents can no longer state with certainty whether rescreening was necessary after the first hearing screening, when rescreening was performed, and when the first hearing device was finally provided. However, it was decided not to ask for the exact result of the first screening (“pass/refer”) as it was feared that parents might then stop completing the questionnaire. As the children were between 4 and 14 years old at the time of the survey, it cannot be ruled out that there were still gaps in the documentation following the outcomes of UNHS, especially in the older children, so that the late recording can be attributed to processes that were not yet optimal at this early stage. However, more recent evaluations of the UNHS show that the refer rate has deteriorated after screening and that the defined target criteria have not yet been achieved throughout Germany [1]. Despite very positive developments such as constantly working on earlier diagnosis, it can therefore be assumed that even today there are still children who are detected late and who are missed out for different reasons.
It should also be mentioned that parents were free to decide whether or not they wanted to take part in the survey. It can be assumed that there were distortions in the sample, as certain target groups could not be reached. These include, for example, families whose first language is not German or who are unable to complete a written questionnaire. A lack of Internet access or an out-of-date e-mail address can also mean that parents did not find out about the survey. Parents who are under particular strain are also unlikely to have taken part in the survey, as are parents who cannot remember the exact dates of hearing device provision/onset of early intervention, etc. In addition, parents with a high level of education were overrepresented in the study.
For future studies, it is recommended that the parents’ data be collected, e.g. through a survey (interview) within the framework of an early intervention, at school, or at the Cochlear Implant Center and at the same time be compared with the data from the available files. In this way, a more representative sample could also be achieved.
Conclusion
The results of this retrospective study show that, despite the UNHS results, there is no guarantee that children will actually be identified early, receive early hearing care and early intervention. In particular, children with unilateral and mild hearing loss appear to be more at risk of being detected late. According to the results of the parent survey, also children with severe-to-profound hearing loss are not always identified early and provided with hearing devices or early intervention. This clearly highlights the need for effective and sustainable tracking procedures and training for professionals, as well as – with regard to late-onset hearing loss – the need to carry out further hearing tests after passing the UNHS, as this is by no means a “hearing guarantee” for further development. Parents and professionals can be lulled into a false sense of security after UNHS [27]. Children with a particular risk of late-onset hearing loss (e.g. with a cytomegalovirus infection during pregnancy) should be monitored continuously. Possible lifelong disadvantages can only be counteracted by early diagnosis as well as early onset of hearing care and early intervention, which provides a wide range of (additional) educational opportunities, including early sign language access and AAC methods.
Notes
Competing interests
The authors declare that they have no competing interests.
References
[1] Brockow I, Söhl K, Hanauer M, Heißenhuber A, Marzi C, Am Zehnhoff-Dinnesen A, Matulat P, Mansmann U, Nennstiel U. Neugeborenen-Hörscreening in Deutschland – Ergebnisse der Evaluationen 2011/2012 und 2017/2018 [Newborn hearing screening in Germany – results of the 2011/2012 and 2017/2018 evaluations]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2023 Nov;66(11):1259-67. DOI: 10.1007/s00103-023-03779-0[2] Nennstiel U, Brockow I, Hanauer M, Heißenhuber A, Marzi C, Söhl K, am Zehnhoff-Dinnesen A, Matulat P, Mansmann U. Endbericht zur Folge-Evaluation des Neugeborenenhörscreenings 2017/2018 im Auftrag des Gemeinsamen Bundesausschusses. Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit: Erlangen; 2022. Available from: https://www.g-ba.de/downloads/40-268-9045/2022-11-17_Kinder-RL_Abnahme-Endbericht-Folge-Evaluation-NHS_Bericht.pdf
[3] Wake M, Ching TY, Wirth K, Poulakis Z, Mensah FK, Gold L, King A, Bryson HE, Reilly S, Rickards F. Population Outcomes of Three Approaches to Detection of Congenital Hearing Loss. Pediatrics. 2016 Jan;137(1):e20151722. DOI: 10.1542/peds.2015-1722
[4] Netten AP, Rieffe C, Theunissen SC, Soede W, Dirks E, Korver AM, Konings S, Oudesluys-Murphy AM, Dekker FW, Frijns JH; DECIBEL Collaborative study group. Early identification: Language skills and social functioning in deaf and hard of hearing preschool children. Int J Pediatr Otorhinolaryngol. 2015 Dec;79(12):2221-6. DOI: 10.1016/j.ijporl.2015.10.008
[5] Tomblin JB, Harrison M, Ambrose SE, Walker EA, Oleson JJ, Moeller MP. Language Outcomes in Young Children with Mild to Severe Hearing Loss. Ear Hear. 2015 Nov-Dec;36 Suppl 1(01):76S-91S. DOI: 10.1097/AUD.0000000000000219
[6] Yoshinaga-Itano C, Sedey AL, Wiggin M, Chung W. Early Hearing Detection and Vocabulary of Children With Hearing Loss. Pediatrics. 2017 Aug;140(2):e20162964. DOI: 10.1542/peds.2016-2964
[7] Ching TYC, Dillon H, Marnane V, Hou S, Day J, Seeto M, Crowe K, Street L, Thomson J, Van Buynder P, Zhang V, Wong A, Burns L, Flynn C, Cupples L, Cowan RS, Leigh G, Sjahalam-King J, Yeh A. Outcomes of early- and late-identified children at 3 years of age: findings from a prospective population-based study. Ear Hear. 2013 Sep;34(5):535-52. DOI: 10.1097/AUD.0b013e3182857718
[8] Ching TYC, Dillon H, Leigh G, Cupples L. Learning from the Longitudinal Outcomes of Children with Hearing Impairment (LOCHI) study: summary of 5-year findings and implications. Int J Audiol. 2018 May;57(sup2):S105-S111. DOI: 10.1080/14992027.2017.1385865
[9] Wong CL, Ching TYC, Cupples L, Button L, Leigh G, Marnane V, Whitfield J, Gunnourie M, Martin L. Psychosocial Development in 5-Year-Old Children With Hearing Loss Using Hearing Aids or Cochlear Implants. Trends Hear. 2017 Jan-Dec;21:2331216517710373. DOI: 10.1177/2331216517710373
[10] Lederberg AR, Schick B, Spencer PE. Language and literacy development of deaf and hard-of-hearing children: successes and challenges. Dev Psychol. 2013 Jan;49(1):15-30. DOI: 10.1037/a0029558
[11] Spencer PE, Marschark M. Evidence-Based Practice in Education Deaf and Hard-of-Hearing Students. New York, NY: Oxford University Press; 2010.
[12] Hintermair M. Psychosocial Development in Deaf and Hard-of-Hearing Children in the Twenty-first Century: Opportunities and Challenges. In: Marschark M, Tang G, Knoors H, editors. Bilingualism and Bilingual Deaf Education. New York, NY: Oxford University Press; 2014. p. 152-86. DOI: 10.1093/acprof:oso/9780199371815.003.0007
[13] Walker EA, Holte L, Spratford M, Oleson J, Welhaven A, Harrison M. Timeliness of service delivery for children with later-identified mild-to-severe hearing loss. Am J Audiol. 2014 Mar;23(1):116-28. DOI: 10.1044/1059-0889(2013/13-0031)
[14] Laugen NJ. Psychosocial Development of Hard-of-Hearing Preschool Children: Implications for Early Intervention. In: Knoors H, Marschark M, editors. Evidence-Based Practices in Deaf Education. Oxford, NY: Oxford University Press; 2019. p. 437-54. DOI: 10.1093/oso/9780190880545.003.0019
[15] van de Sand H, Pützer E, Filip J, Marschall U, Meyer I, Schäfer K, Schubert I. The Frequency of Peripheral Hearing Impairment in Children and Adolescents as Determined From Routine Health Insurance Data. Dtsch Arztebl Int. 2023 Jun 30;120(26):461-2. DOI: 10.3238/arztebl.m2023.0033
[16] Anthes L. Falsche Erinnerungen: Wie verlässlich ist unser Gedächtnis? In: Spectrum.de SciLogs. 2023 Oct 10 [last accessed 2023 Dec 19]. Available from: https://scilogs.spektrum.de/hirn-und-weg/falsche-erinnerungen-wie-verlaesslich-ist-unser-gedaechtnis/
[17] Shaw J. Das trügerische Gedächtnis. Wie unser Gehirn Erinnerungen fälscht. München: Hanser; 2016. DOI: 10.3139/9783446448926
[18] Dale PS, Bates E, Reznick JS, Morisset C. The validity of a parent report instrument of child language at twenty months. J Child Lang. 1989 Jun;16(2):239-49. DOI: 10.1017/s0305000900010394
[19] Fitzpatrick EM, Whittingham J, Durieux-Smith A. Mild bilateral and unilateral hearing loss in childhood: a 20-year view of hearing characteristics, and audiologic practices before and after newborn hearing screening. Ear Hear. 2014 Jan-Feb;35(1):10-8. DOI: 10.1097/AUD.0b013e31829e1ed9
[20] Rohlfs AK, Friedhoff J, Bohnert A, Breitfuss A, Hess M, Müller F, Strauch A, Röhrs M, Wiesner T. Unilateral hearing loss in children: a retrospective study and a review of the current literature. Eur J Pediatr. 2017 Apr;176(4):475-86. DOI: 10.1007/s00431-016-2827-2
[21] Fitzpatrick EM, Dos Santos JC, Grandpierre V, Whittingham J. Exploring reasons for late identification of children with early-onset hearing loss. Int J Pediatr Otorhinolaryngol. 2017 Sep;100:160-7. DOI: 10.1016/j.ijporl.2017.06.039
[22] Yoshinaga-Itano C, DeConde Johnson C, Carpenter C, Stredler Brown A. Outcomes of Children with Mild Bilateral Hearing Loss and Unilateral Hearing Loss. Semin Hear. 2008;29(2):196-211. DOI: 10.1055/s-2008-1075826
[23] Dedhia K, Kitsko D, Sabo D, Chi DH. Children with sensorineural hearing loss after passing the newborn hearing screen. JAMA Otolaryngol Head Neck Surg. 2013 Feb;139(2):119-23. DOI: 10.1001/jamaoto.2013.1229
[24] Fortnum H. Epidemiology of permanent childhood hearing impairment: Implications for neonatal hearing screening. Audiological Medicine. 2003;1(3):155-64. DOI: 10.1080/16513860310001997
[25] Weichbold V, Nekahm-Heis D, Welzl-Mueller K. Universal newborn hearing screening and postnatal hearing loss. Pediatrics. 2006 Apr;117(4):e631-6. DOI: 10.1542/peds.2005-1455
[26] Holzinger D, Weishaupt A, Fellinger P, Beitel C, Fellinger J. Prevalence of 2.2 per mille of significant hearing loss at school age suggests rescreening after NHS. Int J Pediatr Otorhinolaryngol. 2016 Aug;87:121-5. DOI: 10.1016/j.ijporl.2016.06.006
[27] Mann T, Cuttler K, Campbell C. Newborn hearing screens may give a false sense of security. J Am Acad Audiol. 2001 Apr;12(4):215-9; quiz 220-1. DOI: 10.1055/s-0042-1745599




